(Lecture 7-8) Transport of Water & Solutes
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
The solutes that move across cell membranes and epithelia are chemically _____, and mechanisms used to cross are _____.
diverse, numerous
what is energy
ability to do work
what is energetics
energy transfer between systems
what are the 2 main forms of energy
- potential = trapped energy
- kinetic = energy of movement
define diffusion
the net movement of anything generally from a region of higher concentration to a region of lower concentration.
what are the 2 aspects of diffusion that govern many biological processes (i.e. how does it relate to energetics?)
1. lead to a random distribution of molecules (kinetic)
2. tendency of molecules to diffuse is a source of energy (potential)
what is simple diffusion
the movement of solutes or water, gasses dissolved in water, or heat across a gradient
why don't large animals like us rely solely on diffusion?
because diffusion is notoriously slow
what can animal do to increase the rate of diffusion? decrease rate of diffusion?
- increase: increase permeability, increase concentration gradient, decrease distance, increase surface area
- decrease: vice versa
Gradients are a form of energy _____.
storage
chemical vs electrical gradient
- chemical: difference in concentration across a membrane
- electrical: difference in charge across a membrane
Which gradient is important for the diffusion of a neutral solute?
Which gradient is important for the diffusion of a charged/polar solute?
- neutral: chemical only
- charged/polar: both chemical & electrical
what is an electrochemical gradient?
- how does it influence the diffusion of a particular ion
= difference in charge & electrical gradient
- influence: (review electrochemical potential difference equation)
- when C & E gradient are opposite direction: if C>E, ion will move. if C
how to interpret the electrochemical potential difference value Δμ?
if Δμ>0, then
- ΔC & ΔE same direction
- ΔC > ΔE of opposite direction
- move from outside to inside
if Δμ<0, then
- ΔC & ΔE same direction
- ΔC < ΔE of opposite direction
- move from inside to outside
if Δμ=0, then
- the concentration gradient is balanced. The ion is at its equilibrium potential.
(T/F) All ions have the same equilibrium potential
False. Each ion has its own equilibrium potential, which can be calculated using the Nernst equation.
What is resting membrane potential Vm?
- the amount of negative charge found on the inside of the cell membrane when the concentration gradient and the electrical gradients for all ions with open channels are equal
what are the 2 factors required to establish a potential difference across a membrane?
- concentration gradient
- a membrane that is permeable to that ion
what maintains electrical and chemical gradients and delays diffusion?
- active transport
2 main functions of the resting membrane potential Vm
1. provide energy for membrane transport
2. changes in membrane potential used by cells in cell-to-cell signaling
Are cell membranes at equilibrium? Why or why not?
No. Because they have varying permeability and multiple ion gradients.
What is the Goldman equation used for?
Which ions is Vm most dependent upon?
- accounts for permeability & multiple ions
- Na+, K+, Cl-
How do excitable cells (e.g. neuron, muscles) send signals?
- they alter the permeability of their membranes to generate changes in membrane potential to send signals
The resting membrane potential of neurons is ____ (pos/neg).
negative
depolarization vs. hyperpolarization
- depolarization = gets more positive than the resting potential (pos. ion move in)
- hyperpolarization = gets more neg than the resting potential (pos ions move out)
what affects the permeability of hydrophobic solutes (and gases)?
- dependent of factors that affect diffusion through lipids (e.g. molecular size of solute)
- b/c hydrophobic molecules are lipophilic
what affects the permeability of inorganic ions (and water)?
- permeability depends on the number of channels and how many are open
- b/c they are not able to diffuse directly across the bilayer due to being lipophobic
passive diffusion
- types of molecules they transport
- nature of the carriers
- the direction of the transport
- role of energy
- lipid soluble (hydrophobic) molecules
- no transporters needed
- high --> low concentration (steeper gradient = faster rate)
- no energy needed
facilitated diffusion
- types of molecules they transport
- nature of the carriers
- the direction of the transport
- role of energy
- hydrophilic molecules
- facilitated by a protein transporter
- high --> low
- no energy needed
3 types of protein carriers
1. ion channels: small pores for specific ions. "gated" channels
2. porins: like ion channels, but for larger molecules
3. permeases: changes shape to move substrate from one side to the other
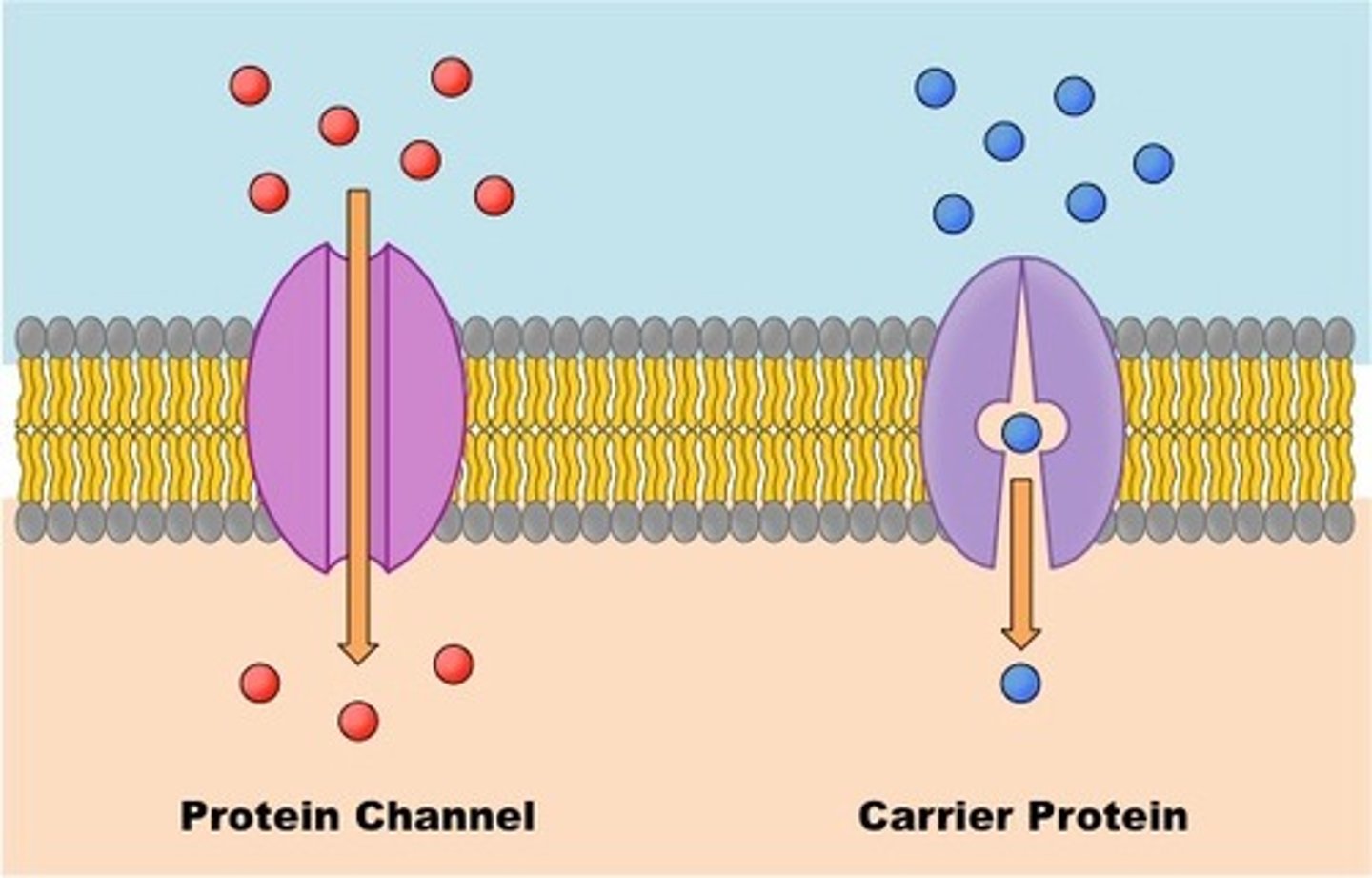
why are ion channels gated
Ion channels are gated to open and close transiently to allow a certain number of ions to pass through them in response to a stimulus.
what are the 3 different ways in which ion channel gates can be opened?
1. voltage gated: open when a certain voltage is reached
2. ligand gated: open when a ligand (chemical messenger) binds the channel
3. mechanogated: opens when a mechanical stimulus is present
active transport
- types of molecules they transport
- nature of the carriers
- the direction of the transport
- role of energy
- used to accumulate needed molecules.
- protein transporter is needed
- from low to high concentration (against gradient)
- hydrolysis of ATP provides energy
primary vs. secondary active transport
Primary - transport directly coupled with ATP
Secondary- ATP first used to create a gradient and the potential energy is used to drive something across membrane
what are the 2 types of secondary transporter
- symport/contransporter: same direction
- antiport/exchanger: opposite direction
how is water transported? does it require energy?
via aquaporins. no energy required
what is osmosis
passive transport of water from the solution of lower osmotic pressure to the solution of higher osmotic pressure
how is passive water transport controlled?
by active solute transport
Solute form ____ bond with water molecules
hydrogen bonds
how do solutes affect the colligative properties of water
(tip: think about putting salt in water, and how that affects its properties)
- freezing point
- boiling point
- vapor pressure
- osmotic pressure
- decrease freezing point
- increase boiling point
- increase vapor pressure
- increase osmotic pressure
What does water's colligative properties depend on?
- number of solutes? size? charge?
- number of solutes
what is a hydration shell
forms when solute is surrounded by water molecules