Synthesising Polymers
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
What are the two primary methods of synthesis
Chain growth, and Step Growth
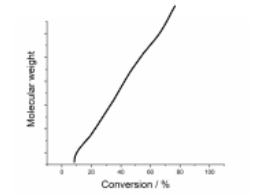
Describe Chain growth Polymerisation and Draw a graph displaying this
Monomers are added 1-by-1 at the end of a growing polymer chain
Length is dictated by the number of growing chains, and grows linearly over the course of the polymerisation as the monomers are consumed
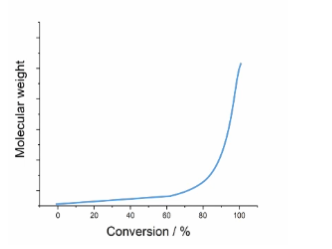
Describe Step growth Polymerisation and draw a graph displaying this
Both monomers and growing chains have reactive groups, all species can join to form longer chains
Monomer is quickly consumed, but it is not until high conversions that long polymers are produced as growing chains join together
Name and describe the 3 steps of chain growth Polymerisation
Initiation – reactive species is formed and reacts with the first monomer to generate a reactive polymer end group
Propagation – the reactive end group reacts sequentially with further monomers, leading to extension of the chain
Termination – Something happens to deactivate the reactive end group, stopping polymerisation
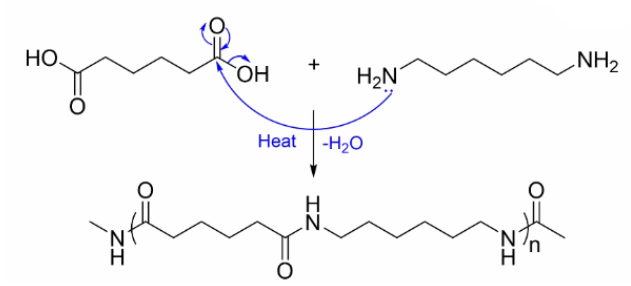
Using a hydrocarbon containing carboxylic acid and an amine, draw an example of Step-growth
What are the three main methods of Polymerisation
Free Radical
Cationic
Anionic
Which polymerisation can also be used for the ring opening of cyclic monomers
Anionic Polymerisation
Describe why we use free radicals
Free radicals possess unpaired valence electrons which make them very reactive.
They can therefore react with a wide range of functional groups, as such, they can be used to polymerise alkenes.
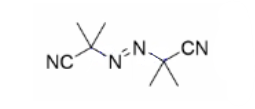
Draw the three steps of Free radical polymerisation using this as an example
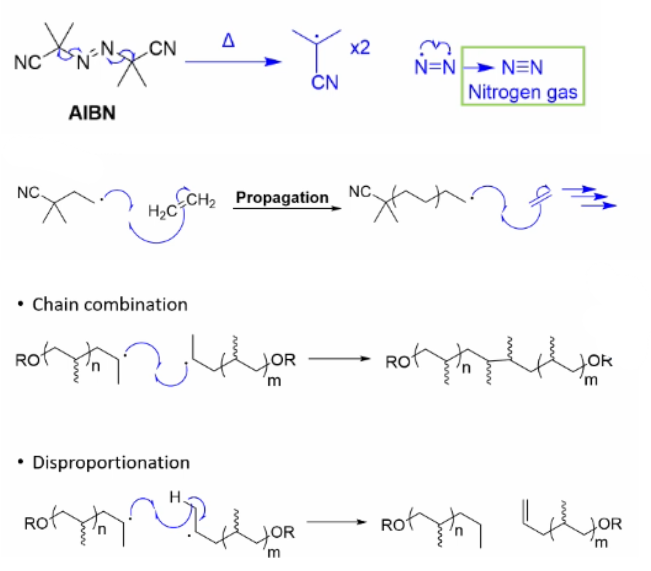
Describe the initiation step of free radical polymerisation and the importance of the initiator
Activated initiator can react with a monomer to generate the reactive polymer chain.
An ideal initiator should be stable at room temperature, but break down to form reactive radicals when heated or irradiated with UV light.
Describe the propagation step of free radical polymerisation, comment on the relation between speed of this step and dispersion
Radicals on the polymer chain add to another monomer unit, extending the chain and moving the reactive radical to the end, ready to undergo the same process again.
These processes can be incredibly fast, creating chains of 1000s of monomers in as little as 1 second.
But this speed can come at the cost of control, leading to polymers with high Ð.
Describe the termination step outlining the two main mechanisms
Propagation and chain extension will continue until something acts to quench the reactive radical. There are two main mechanisms of termination:
Chain combination, where 2 reactive polymer chains join together.
Disproportionation, where a proton is abstracted from another growing polymer chain, creating one polymer alkane and one polymer alkene.
What are other examples of processes that can stop propagation in Free Radical polymerisation
chain transfer to solvent or monomer, inhibition with oxygen, or quenching with initiator.
Draw out the Mechanism for Cationic Polymerisation using this
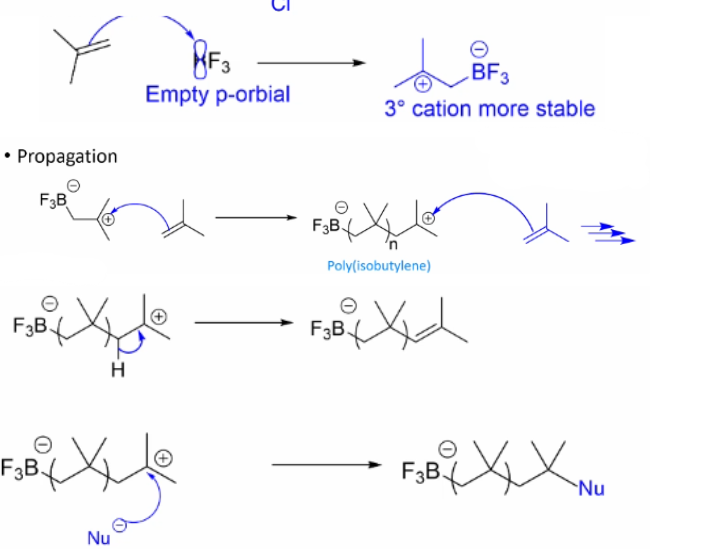
Describe how we use Cations for polymerisation
In cationic polymerisation, an electrophilic polymer reacts with monomer units to generate a cationic polymer chain that can undergo propagation.
Particularly successful for the polymerisation of alkenes that have electron donating groups that can stabilise the carbocation
Describe the initiation stage of cationic polymerisation, outline what acids are most commonly used and why.
Uses Acids as initiating electrophiles to undergo electrophilic addition of H+ breaking the double bond and forming a carbocation.
Most commonly uses Lewis acids as initiating electrophiles. Brønsted acids can be used but the anion must be a poor nucleophile to prevent normal Markovnikov addition to the alkene.
Describe the Propagation stage of cationic polymerisation and state what the monomers reactivity is dependant on/
cations on the polymer chain react with monomers leading to chain extension. Monomer reactivity depends on ability to stabilise the cation.
Describe what can cause the termination stage of the Cationic
Can be caused by the loss of a proton from the growing chain. Alternatively, the chain can react with a nucleophile, stopping the growing of a polymer at a specific point.
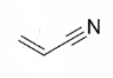
Draw out the mechanism for anionic polymerisation using this is the monomer
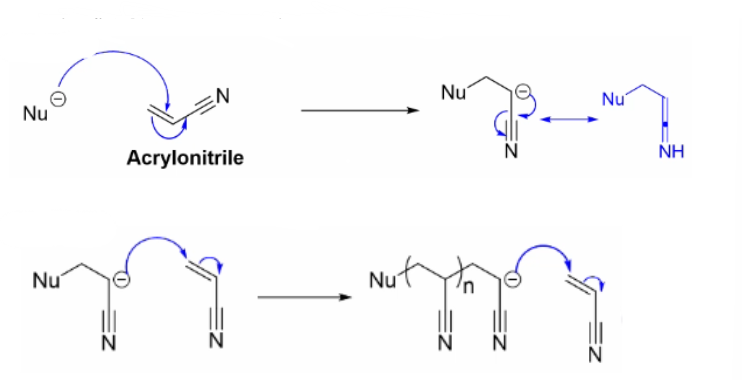
Describe how we use Anion to form polymers
a nucleophilic polymer reacts with electrophilic monomer units to generate an anionic polymer chain that can undergo propagation.
Nucleophiles do not usually add to alkenes, and so a strong electron withdrawing group is required to polymerise these monomers.
Describe the initiation stage of Anionic Polymerisation, and describe how the reactivity of the monomer pairs it with a nucleophile
Nucleophilic addition to create a nucleophilic polymer via the cleavage of a double bonded carbon.
For monomers with low reactivity, strong nucleophiles such as butyl lithium (BuLi) are required. More reactive monomers can be activated using weak nucleophiles such as amines.
Describe the propagation stage of anionic polymerisation
Anions on the nucleophilic polymer chain donates donate an electron to the carbon double bond of the electrophilic monomer breaking the carbon double bond, leading to chain expansion
Describe the termination stage of Anionic polymerisation
When exceptionally pure solvents and reagents are used, anionic polymerisation does not undergo termination. Polymerisation will continue until all monomer is consumed and the polymerisation is living.
What is living polymerisation
A class of chain growth polymerisation, in which, there is no termination. The reactive polymer end group is therefore ‘living’ and will continue to react with monomer till it is all consumed.
Compare rate of initiation and rate of propagation in living polymerisation
Rate of initiation is far greater than propagation
Generally what is the dispersity value of living polymerisation
lower than 1.1, due to all chains growing simultaneously at a uniform rate,resulting in a narrow distribution.
Which types of polymerisation can be living
All anionic is living
Cationic and free-radical can be made living via careful control of conditions
How to calculate the average chain length for living polymers

Describe what Statistical copolymers are
If multiple monomers are present during polymerisation then the distribution of the monomers will be essentially random creating a sequence following predictable statistical rules,
though can be influenced by the relative reactivity of each monomer.
Describe what the Block Copolymers are
Living polymerisations with precisely defined segments of each monomer.This is due to chains bing chemically linked via a covalent bond but still being incompatible with each other.
As such, they attempt to separate but cannot due to the covalent bond, leading to them forming ordered nano structures.
Describe formation of Branching polymers
In free radical polymerisation if we abstract a hydrogen atom from the middle of another polymer rather than disproportionating we form a branched polymer
Describe cross linking polymers
This can take place during of after polymerisation, and sees individual polymer chains linking together via bonding to create a large 3D network
What is tacticity
refers too the stereochemical arrangements of polymers
Name the 3 tacticity’s of polymers and which type of polymerisation undergoes them
Atactic polymer: The orientation of the functional group is random (Radical
Isotactic polymer: All functional groups are presented on the same side of the polymer backbone. (catatonic and anionic)
Syndiotactic polymer: Functional groups alternate in a regular manner on both sides of the polymer. (catatonic and anionic)