Chemistry U3 AOS1 (Chapters 2, 3, 4,5)
1/90
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
91 Terms
Fuel
Is a substance with stored energy that can easily be released for heat/power.
By reacting usually with oxygen- a combustion reaction. Heat released which is how we get energy to use.
Can be transported and stored safely.
Used mainly for transport, heating, etc.
Chemical reactions
Involve a change in energy due to breaking of reactant bonds and forming new ones as products. This is the system.
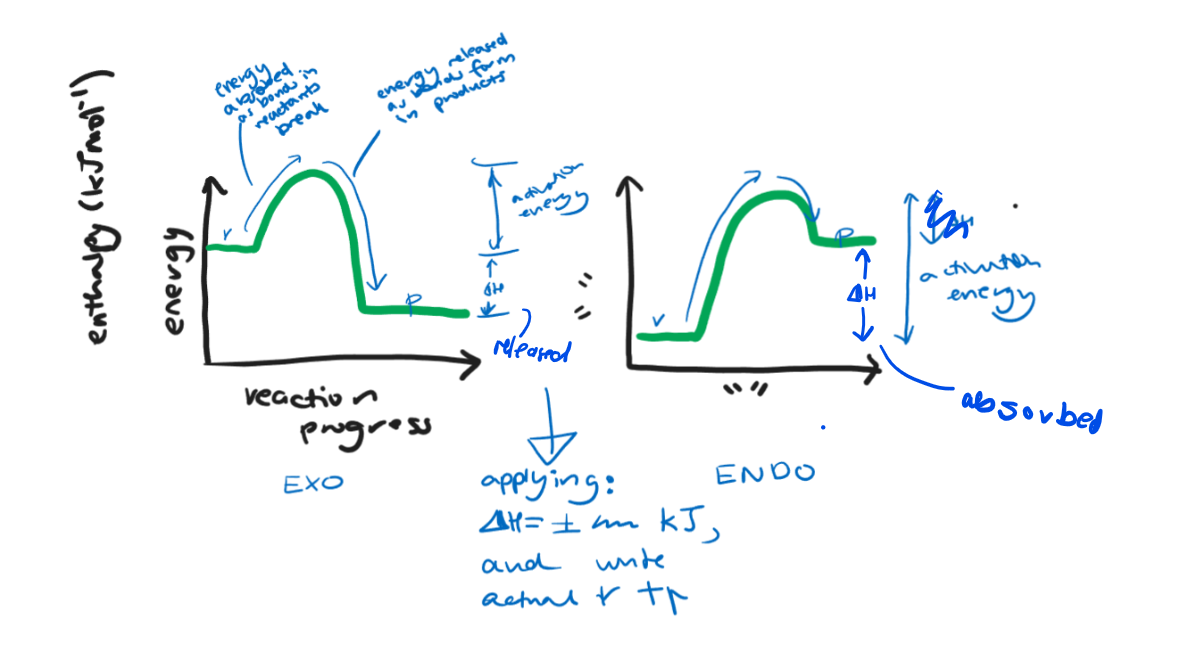
Activation energy
Energy required to break reactant bonds for reaction to continue, so reactions still first absorb some energy
Chemical energy
Of a substance is its heat content/enthalpy (Chemical potential, total chemical energy. ). Net enthalpy change in a reaction is ∆ H (this is the exchange of hear energy between system and surroundings, under constant pressure, which = Hp - Hr) In joules.
Exothermic and Endothermic Reactions
Exothermic have net release of energy to surroundings, so ∆ H is negative. More energy was absorbed to break reactant bonds than was used to form products’ bonds. There is an excess of energy, an increase in temperature.
Opposite for endothermic.
Thermochemical equation
Has the equation but also the net enthalpy change.
Energy profile diagrams
Represent energy changes in a reaction.
Non-renewable and renewable fuels
A fuel is considered non-renewable if it cannot be replenished at the rate at which it is consumed.
Fossil fuels
Are non-renewable fuels.
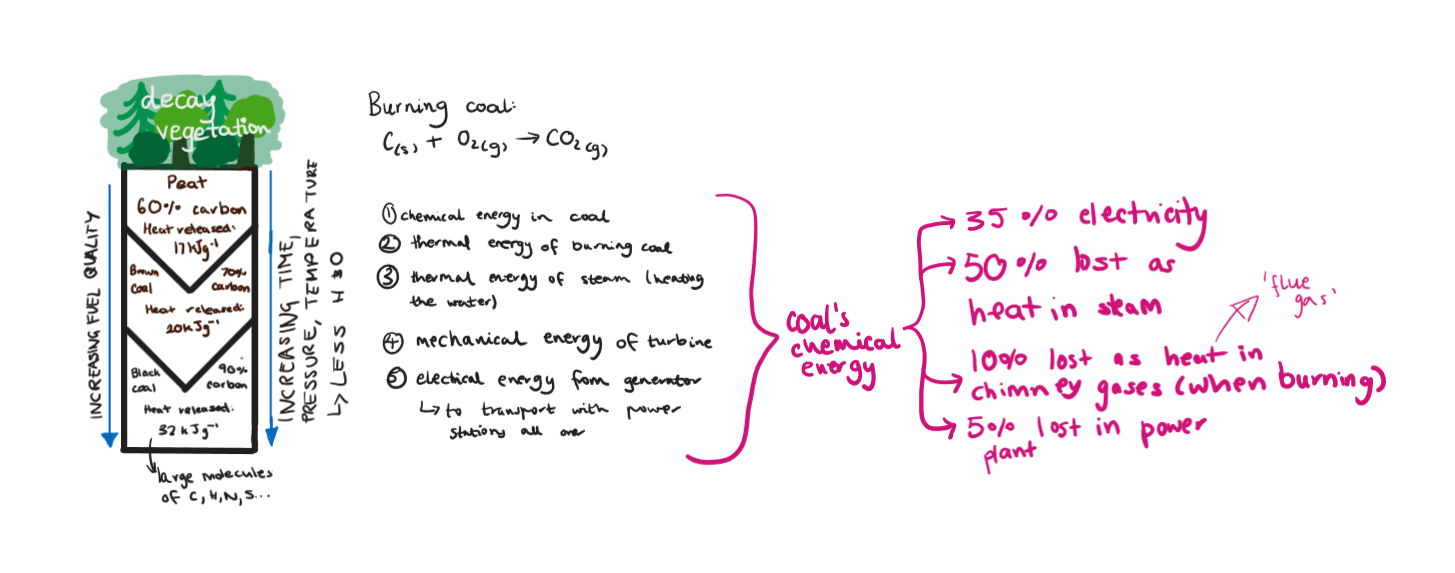
Coal, oil/petrol/petrodiesel, natural gas.
Are formed from ancient plants/animals/microorganisms buried under tonnes of mud/sand/rock. Complex changes occur to become fossil fuels over milions of years (hence no sustainable). The matter still has some of the chemical energy it had collected from photosynthesis. Can be considered as trapped solar energy.
70% of our electricity.
Coal
Petrol/petrodiesel/oil
Crude oil/ petroleum.
Carbon-basded life decomposes and forms crude oil/petroleum after high pressures and temperatures and millions of years.
Mixture of hydrocarbons (mostly those of the alkane homologous series). Useful compounds are separated and used or processed further to be more specific.
Petrol is one of the fractions, and contains octane and other alkane of similar boiling points.
2C8H18(l) + 25O2(g) → 16CO2(g) + 18H2O(l)
This combustion happens in cylinder of car engine. Hot gases formed push pistons in engine up and down.
Petrodiesel is also a fraction but has longer molecules.
Natural gas
In Earth’s crust.
Mainly methane but also ethane, propane. Perhaps, H2O, S, CO2.
May be in gas reservoirs trapped between rock layers, part of petroleum deposits, coal deposits by being bonded to surface of coal (coal seams tend to have water, so its pressure can keep gas adsorbed to coal surface; this is coal seam gas (CSG).
It is drilled out if between rock layers or in petroleum deposits.
Exported as liquid ((LNG). Liquid conversion increases energy density, but liquification consumes a significant amount of energy,
Use in Victoria to generate electricity for power grid, but also via pipes to cook. Hot gases produced by (combustion of methane) burning these small alkines cause air to expand in a combustion turbine to generate electricity.
Biofuels
Biochemical fuels. Come from plant materials like sugarcane, grass, waste). Can be mixed with fossil fuels. Meet future energy needs, less fossil fuel impact.
Biogas
Formed by the anaerobic breakdown of organic waste. Burnt (CH40 to generate electrical energy (not much is produced as there is less methane than in natural gas, so less energy).
Anaerobic bacteria decompose complex molecules like carbohydrates and proteins into smaller ones (CO2, CH4) for heat and power to run the farm. it occurs in a digester (tank), and the waste becomes fertiliser.
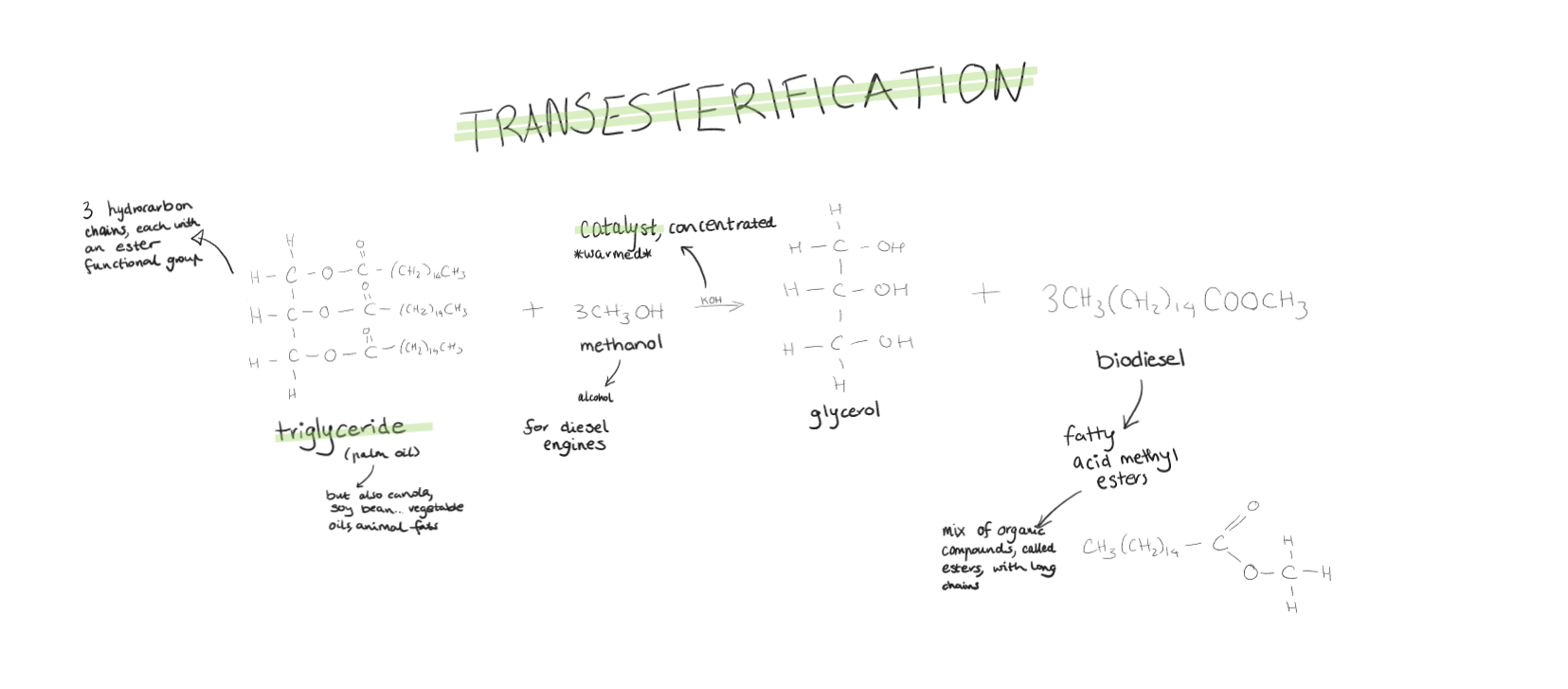
Biodiesel
Produced from triglycerides in animal fats or plant oils. The triglycerides react with methanol is a transesterification reaction to form biodiesel.
Comparing biofuels
Advantages…
CO2 is absorbed in growth of crops that are used (via photosynthesis, to glucose and then converted into glucose and starch) as fuel, can be replenished, produced from material hat would else have been waste.
So theoretically carbon neutral. But in practice there is also energy needed to farm, fertilise and transport.
CO2 is released against when burnt but the absorbance means there is less net impact.
But, shift to their large-scale production could strain resources and available farmland; leading to land degradation, clearing, food supply struggles. Wind and solar is biggest in such impacts.
Photosynthesis
Green plants use energy from the Sun, which is then stored in glucose molecules, for photosynthesis.
It is an endothermic reaction occurring in the chloroplasts of cells of green leaves. It is so plants can make their own food- glucose. They also use glucose as a monomer for polymers like cellulose and starch.
ΔH= +2803kJ
Cellular respiration
Plants and animals use cellular respiration (analogous to combustion of fuels) to oxidise glucose to obtain energy.
C6H12O6(aq) + 6O2(g) → 6CO2(g) + 6H2O(l) ΔH= -2803kJ
Glucose is the primary energy source- mainyl in sap, blood, tissue.
Done so mainly via aerobic respiration, as O2(g) is needed.
It is not just the reverse of photosynthesis, it is more complex than that.
We need this energy for warmth; movement; synthesis of biomolecules like hormones, enzymes, carbohydrates, tryglycerides; and functions like breathing…
Starch and glucose are more rapidly so they are the main sources of energy.
Nutrients
Substances used by an organism to survive, grow, reproduce. Attained via food.
Metabolism in the human body
Metabolism is where the energy released as these molecules are digested back to smaller units.
Of carbohydrates, fats and proteins. They are the source of energy in animals.
Carbohydrates
Cx(H2O)y
During digestion, enzymes (organic catalysts that alter rate of biochemical reactions) in saliva and small intestine break start molecules (polymers) back to glucose.
Glucose is transported via blood to cells where respiration can occur. Energy is required to form the bonds of large carbohydrates, then released in digestion.
Fats and oils
They are triglycerides- large non-polar molecules with three long hydrocarbon chains attached to a glycerol molecule. Fats provide and store energy in the body. Digestion breaks it down and components of that can be oxidised in the body’s cells to CO2 and H2O, releasing a lot of energy.
Glucose in humans is burtn in muscle cells for energy, forming glycogen.
Protein
They are rarely used as energy. If intense exercise depletes glycogen and fat, then.. yes. Must soon be replaced to ensure functioning.
Energy content and value
Energy content is the amount of energy a food/fuel can supply. In kJ g-1, kJ/100g, or kJ mol-1 if a pure substance like glucose.
Heat of combustion.
Varies within the group, too.
Energy value is the energy available to the body from a nutrient/food. (so, the actual)
The human body will often obtain less energy from a food item than the theoretical quantity based on direct combustion of the food.
Which nutrient groups have the higher energy value?
Fats produce the most energy per gram of the major food groups, then proteins and finally carbohydrates (39, 24, then 16).
Same, as in energy value (37, 17, then 16 kJ g-1).
Fats and oils have a higher energy values because there is greater potential for oxidation and hence to release more energy on combustion. Because carbohydrates have more oxygen atoms, it is already partially ‘oxidised’, with the carbon atoms having a higher ‘degree of oxidation’.
When food is burned, there is more energy obtained than when digested. Dietary fibre is mainly of the carbohydrate cellulose, and we cannot digest most fibre, so we can’t get that energy.
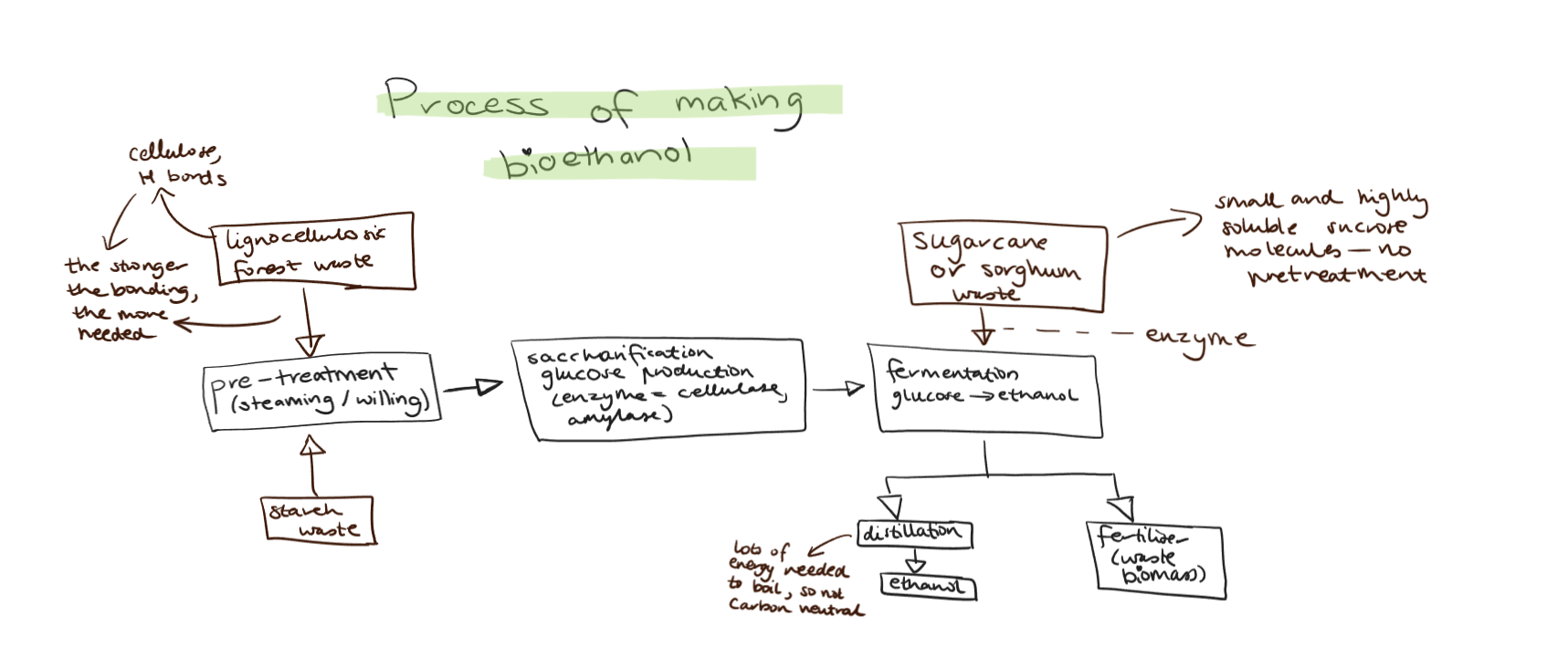
Bioethanol
Can be produced by fermentation of carbohydrates and sugars.
Fermentation is where enzymes and microorganisms catalyse/facilitate reactions involved at 35oC- if higher they are destriyed. Glucose is in plants and larger carbohydrates, which is broken back down to glucose. That carbohydrates are pulped with water (biomass blended to break it up). Enzymes break down to smaller sugars then glucose. Enzymes catalyse the fermentation of glucose to make ethanol solution.
C6H12O6(aq) → 2CH3CH2OH(aq) + 2CO2(g)
Bioethanol solutions are distilled to separate the ethanol from the water. They are distilled by using different boiling points of liquids to separate them- ethanol has alower boiling point. The solution is heated to boiling, then into a tall distillation column. It is carefully controlled so that the water is at the bottom and the ethanol gas (79oC) is collected from the top. Leftover water is removed with micro-filtration and dehydrating agents.
Bit of methanol is added (methylated spirits), so they are not consumed.
If water was kept, this could damage engine cylinder, cause corrosion, explosion with fuel injectors (when added to E10).
Carbohydrate sources for ethanol
We have commercial plants producing bioethanol. The carbohydrate sources (needs an abundance of them) used are biomass from sugar cane (high in sucrose), sorghum (high in starch), wheat (also high in starch). And forest waster (high in cellulose). This is good because we have strong farming and forestry industries.
There is plenty of corn and wheat and sugar can, but they’re needed for food! So forest waste. We have a large timber industry. It has lots of cellulose, a polymer of glucose. But it takes a harsh pre-treatment with steaming and specialised enzymes.
E10 against Petrol
Bioethanol is sold as E10, which is petrol blended with up to 10% ethanol.
Used for combustion engines, fuel cells, electricity generators.
Energy density of ethanol is lower than that of petrol.
Reduced fossil fuel needs and emissions of particulates and gases (oxides of nitrogen) but more can damage engines.
C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(l) ΔH= -1360kJ
That’s 62% of the energy content of petrol. So more in mass/volume is needed. This is because carbon atoms are being partly oxidised (burnt) with being already present in some oxygen.
E10 vs Petrol
Lower energy density (46 vs 48 kJ g-1)
Cheaper
Less widely distributed
Cleaner combustion
Renewable
Lower net CO2 emissions (aborbs to negate the combustion)
More CO2 produce per kilometre driven
Generally safer production
Complete combustion
of hydrocarbons and carbon-based fuels containing oxygen produces carbon dioxide and water.
Needs energy to get the process started, but it’s exothermic.
Reacts with oxygen to produces oxides ~ oxidation reaction. In the case of cellular respiration. In redox with oxygen as a reactant, oxidation is the addition of oxygen to form oxides.
We can see by the thermochemical equations that combustion is exothermic.
Doubling the coefficients of the chemical equation of the reaction causes the ΔH value to also double. Twice the reactants to produce/absorb twice the energy. The magnitude of the reaction.
If we reversed the equation, hence reaction, it would be endothermic but of the same magnitude.
Includes states of matter. Changes of state involves enthalpy changes. Boiling water is endothermic, as heat is applied!
H2O(l) → H2O(g) ΔH=+40.7kJ
As a liquid it has a gretaer magnitude than as a gas in combustion. Because being a gas consumes some of that energy endothermically.
H2O(l) in combustion means that ΔH = -5450 kJ
But as H2O(g) the ΔH = -5409.3 kJ !
Incomplete combustion
Happens when it undergoes in a limited supply oxygen. Products include carbon monoxide and/or carbon dioxide, and water.
2CH4(g) + 3O2(g) → 2CO(g) + 4H20 (l)
Not all of the carbon converts to carbon dioxide. Hydrocarbon burns yellow, smokey, sooty flame because of glowing carbon particles.
What to use when comparing furls
Enthalpy, enthalpy chage, molar enthalpy (enthalpy of substance per mole, ΔHc for 1 mol)
Heat of combustion and enthalpy of combustion
Heat of combustion (of fuel, qc) is the heat energy released when a specified amount (1g, 1L, 1mol) of substance burns completely in oxygen. Enthalpy of combustion, ΔHc, which is found in the thermochemical equation, is a negative value which has the same numerical value as the heat of combustion.
Heat of combustion indicates the max. quantity of energy that can be released when a specified amount of fuel undergoes complete combustion. In kJ mol-1 or g-1. Has a positive value, because they’re a measure of quantity of energy released.
Quantity of energy released
E = n * ΔHc (hoc)
This can be used to compare different fuels by using heats of combustion. Heat of combustion tables can be used to determine the enthalpy change, ΔH, in a thermochemical equation.
‘How suitable are they as transport fuels?’ If they have a greater magnitude of ΔH. CO2 present…
Mole only if a pure substance. Wood, coal, kerosene are mixtures of chemicals with no specifie chemical formulas or molar masses, so kJ g-1 or kJ L-1. Energy contet=nt is often in kJ g-1. Divide by molar mass to get kJ mol-1 !
Stoichiometry in combusting fuels
Mole reatio
n=N/Na (6.02 × 10²³ mol)
n=m/M
n=V/Vm (24.8 kJ mol-1 ) if at SLC (100kPa, 25oC)
PV=nRT
T in kelvin (273K = 0oC)
R is the ideal gas factors, 8.31 kJ mol-1
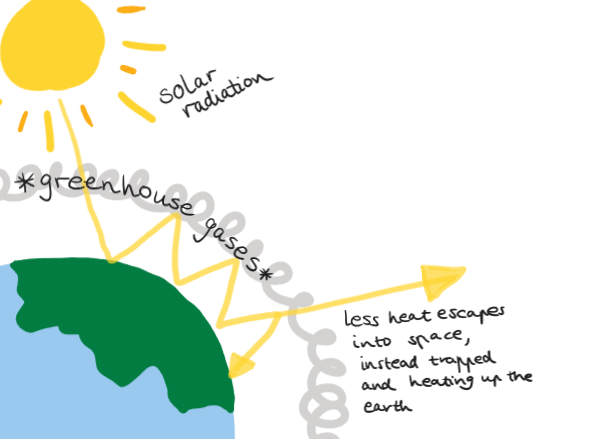
Greenhouse gases produced
Compare fuels by energy released and total greenhouse gases (H2O, CO2) produced. And quantities of chemical required. Quantity of energy obtained from fuel combustion depends on type of fuel amount of fuel, in/complete combustion.
Greenhouse gases can absorb infrared radiation , but increased presence with human activities contributes to the enhanced greenhouse gas effect, causing global warming. Methane is more potent and powerful, which is escaping from landfills, coal mines, agriculture. Capturing methane and using it as biogas does increase CO2 but reduces CH4.
Limiting reactants or reagents
The limiting reactant or reagent mut be idenfitied and used in stoichiometric calculations, if quantities of more than one reactant or reagent is provided for a chemical reaction.
A reactant is a starting material that undergoes change during a chemical reaction whereas a reagent is a substance added to a system to cause a chemical reaction.
The limiting reactant or reagent that is completey consumed in the reaction The other one is hence the excess reactant.
calculate the moles of each reactant
identify which is limiting
a) find the mole ratio
b) find the mole of one of the reactant using mole ratio and the other mole, that is then the mole needed to react with the used mole.
c) If the value is less than the provided mole in the equation, it means that the mole will be the limiting reactant.
d) Determine then the mole reacted of the excess reactant.
use amount of limiting reactant to find amount of produce formed
Specific heat capacity
Of a substance measures quantity of energy (joule) needed to increase the temperature of a specific quantity of that substance (1g) by 1oC.
Water is 4.18 J g-1 oC-1
q (j) = mcΔT
Experimental determination of the heat energy released in the combustion of a fuel
specific heat capacity of water is used to determine the heat energy absorbed by a measured mass of water place above the burning fuel.
heat of combustion (kJ mol-1) of pure substance canbe found using experimental results of energy absorbed by the measured mass of water and the amount of substance (mol) whose combustion released that energy using = q (kJ)/n (mol).
because foods and some fuels are mixtures, energy content (kJ g-1) is used instead.
Limitations and improvements of this
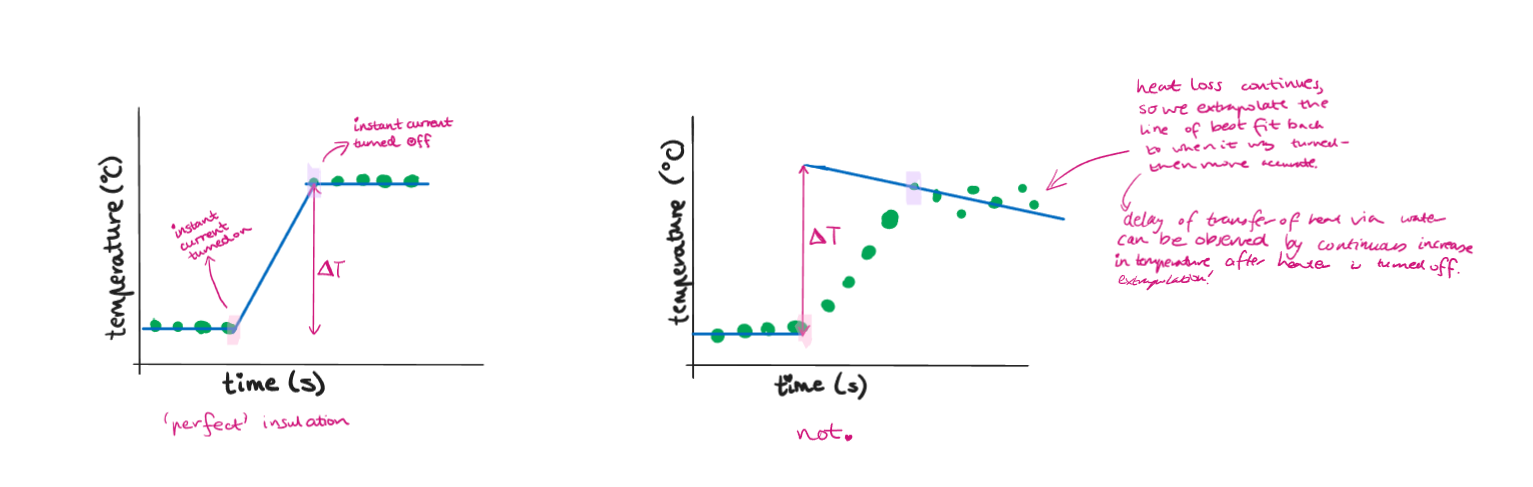
Due to extensive heat loss to surroundings during the experiment (systematic error), calculations of the HoC of pure substance can be inaccurate.
To improve, repeat, insulate, put a lid on the water container, minimise distance of flame and water.
Calorimetry
The experimental method by which the heat energy released/absorbed in a chemical reaction/physical process is measured.
Calorimeter
The instrument that measures energy changes in a reaction. made up of an insulate container of water in which the reaction occurs, with a stirrer and thermometer to measure the temperature change during the reaction.
Solution calorimeter
An insulated container that holds a known volume of water an in which a reaction occurs in the solution, like dissolution of a solid or a neutralisatgion reaction, can be carried out.
Bomb calorimeter
An insulated container (no heat escapes) in which a sealed, O2-filled reaction vessel (‘bomb’) is surrounded by a known volume of water. Combustion takes place in the pressurised veessel and heat from reaction is transferred to surroundings (water). There is an electric heater for the sample, a stirrer, for uniformity, sample in crucible with O2 under pressure, thermometer, electric heater calibrating calorimeter.
Involves gaseous reactants/products.
Calibration
calorimeters are calibrated (beforehand) to establish relationship betweeb the energy transferred to the water and the temperature change un the calorimeter.
It involves a known quantity of heat energy from an electrical or chemical source.
CF= E/ ΔT
For a more accurate ΔT value, use a time-temperature graph as it may be affected by heat loss from calorimeter in calibration.
Electric calibration
CF= E / ΔT
E= VIt
ΔT in temperature (difference is same, if kelvin or celsius)
E in joules
V in V (volts) - voltage
A in A (amps) - current
t in s (seconds)
Calibrated using electric heater to release a known quantity of thermal energy and measuring the resulting temperature of water in calorimeter.
Chemical calibration
CF = E / ΔT
E= n * ΔH (enthalpy of solution)
In joules
Dissolve a known amount (mol) in the calorimeter. The ΔT is measures and used to calculate CF.
Time-temperature graphs
Energy transformation efficiency
Refers to the percentage of available energy that is transformed to the desired form of energy, as found by: (useful energy)/(energy inout) *100
Because when transforming the energy in fuels/foods into a form we can use/digest, much energy is lost in the process.
The total amount of energy does nit change, but it is not all converted into one specific form. Energy is always conserved, but only some of it is likely to be useful.
Energy converters
Convert energy from non/renewable sources into the form we require.
Energy value via energy transformation efficiency
Energy value in kJ g-1.
Energy value of a food can be calculated using data for the available energy of the various components of the food and the percentages of each component of the food.
Remember nutrients, their energy value. How digestion does not release as much energy as when it is burned. May be due to incomplete absorption by the body after digestion, incomplete oxidation of nutrients, heat loss .
Redox reactions
Involve the transfer of electrons from one species to another.
Oxidation is the loss of electrons, and reduction is the gain of electrons, which occurs simultaneously.
Half-equations
Each represent oxidation and reduction. They show what is happening as electrons are transferred in a redox reaction.
Electronegativity
The ability to attract electrons.
Reducing agents
Loses electrons to another substance, causing reduction for the other substance, while itself is oxidised.
Oxidising agents
Gains electrons from another substance, causing oxidation for the other substance, while itself is reduced.
The overall equation
Half-equations are added together to obtain the overall equation. May be necessary to multiply them by a factor to balance the electrons (do not multiply when simply stating the half-equations).
Oxidation numbers
Free elements = 0
Oxygen = -2 | except in peoxides, -1
In ionic compounds of simple ions, oxnos = charge of the ion.
Hydrogen = +1 | when with non-metals
Sum of oxnos in a neutral compound = 0
Sum of oxnos in a polyatomic ion = charge on the ion | Most electronegative element is assigned the oxnos
Transitional metals and some non-metals have variable oxnos.
is increase in oxnos reduction or oxidation?
oxidation
What does no change of oxnos indicate?
The reaction is not redox
Conjugate redox pair
Consists of an oxidising agent (a reactant) / a reducing agent (a product) that is formed when the oxidising agent gains electrons (oxnos decreases).
The other pair… reactant is the reducing agent / product is the oxidising agent that is formed when the reducing agent loses electrons (oxnos increases).
Example: 2K(s) +Cl2(g) → 2KCl(s)
oxidation - K(s) / K+ (s)
reduction - Cl2(g) / Cl-(s)
Steps for balancing redox reactions under acidic conditions
for each half-equation…
balance main elements of the half-equation
balance O by adding H2O(l)
balance H by adding H+(aq)
add electrons to balance charges as required
states!
multiply to balance electrons, and combine to make overral equation, remove electrons. cancel out any H+, OH-, H2O that appear on both sides.
Steps for balancing redox reactions under alkaline conditions, with OH- present in the main elements
for each half-equation…
balance main elements
balance hydroxides with OH-(aq)
balance charge by adding electrons
states!
multiply to balance electrons, and combine to make overral equation, remove electrons. cancel out any H+, OH-, H2O that appear on both sides.
Steps for balancing redox reactions under alkaline conditions, with OH- not present in the main elements
for each half-equation…
balance half-equation, completely, as though it were acidic
add enough OH-(aq) to both sides to neutralise H+(aq)
neutralisation reaction between H+(aq) and OH-(aq) makes water, so cancel out the water molecules of the sides where there are fewer water molecules and reduce the water molecules on the other side by the same number
states!
multiply to balance electrons, and combine to make overral equation, remove electrons. cancel out any H+, OH-, H2O that appear on both sides.
spectator ions

galvanic cell
is a type of electrochemical cell, in which chemical energy is directly converted to electrical energy through a redox reaction- that is spontaneous and exothermic.
electrochemical cell
device in which chemical energy is converted to electrical energy, or vice versa
describe the cell
a cell is made from two half-cells.
each half-cell contains a conjugate redox pair.
oxidation happens in one half-cell and reduction in the other. the electrode, in contact with solution, in the half-cell where the oxidation occurs is called the anode (-), the other is cathode (+).
each half-cell has a half-reaction, electrons flow through the external circuit from anode to cathode, not coming in direct contact.
salt bridge! (below)
current flows because of chemical reaction.
the anode may eventually corrode and the cathode may become covered in a deposit, its solution losing some colour.
battery
connected several cells in series to have a higher potential difference (voltage)
purpose of salt bridge
allows a cell to produce electricity by allowing the movement of ions between the two half-cells. cations in the salt bridge move towards the cathode, anions to anode.
internal circuit
typically filter paper is soaked in a relatively unreactive electrolyte (chemical substance that conducts electrical current) like KNO3.
it balances charges formed in the two compartments. without it, the charges would accumulate and the reaction hence stops.
if the redox pair does not include solid metals?
some redox pairs (Br2(aq)/Br-(aq) and Fe3+(aq)/Fe2+(aq) ) do not involve solid metal, so an inert electrode like graphite or platinum is used.
a gas electrode is used when one is a gas (H+(aq)/H2(g)) . then a glass tube has an inert electrode with an opening for the gas to be added.
if the reactants in a galvanic cell were allowed to come into direct contact
the chemical energy is converted to heat energy, not electrical, going to surroundings
primary/secondary cells
primary cells are not rechargeable. they go flat when equilibrium is reached (where quantities of reactants and products tend to not change). the galvanic cell discharges, cell voltage drops to zero.
the products slowly migrate away from electrodes or are consumed by side reactions occurring in the cells, preventing cells from being recharged.
alkaline cells are primary cells- they are cost-effective, when high currents are needed intermittently, small and designed so that half-reactions can happen separately in one container.
secondary cells are rechargeable.
annotate this diagram
hydrogen half-cell
is used as the standard reference half-cell, its value is arbitrarily assigned as zero (0.00V).
(H+(aq)/H2(g))
H2(g) is the standard hydrogen half-cell electrode
galvanic cells can help compare relative reactivity of metals, determining oxidising and reducing strengths of substances. can then predict products of various reactions, calculate voltage of cells, develop more powerful and long-lasting batteries.
standard electrode potential Eo
or standard reduction potenial…
of a half-cell is measured by connecting the half-cell to a standard hydrogen half-cell and measuring the voltage produce.
you can’t measure a half-cells potential difference on it own, so redox with the standard reference half-cell!
the potential difference, electromotive force (V) with a voltmeter. electromotive force between two points in a circuit- applying it, it exists between two half-cells when a current flows in a galvanic cell due to one half-cell having a greater tendency to push electrons in the external circuit.
gives a numerical measure of the tendency of half-cell reaction to occur as a reduction reaction.
Fe2+(aq) + 2e- <=> Fe(s)
Eo=-0.44V
the electrode was negative when connected to hydrogen half-cell. It (Fe(s)) is hence being oxidised.
The electrochemical series
Standard electrode potential is used as the basis of the electrochemical series. In the electrochemical series, half-reaction are listed in order so that the strongest oxidisng agent is in top left (most positive Eo value) and the strongest reducing agent is bottom right (most negative Eo value).
It is valid for SLC (including concentration 1M).
relative strengths of oxidising agents and reducing agents can be compared using Eo.
Maximum potential difference
Of a cell under SLC
= E^o of half-cell with oxidising agent - E^o of half-cell with reducing agent
for a spontaneous (‘naturally occurring’) reaction to occur, an oxidising agent must react with reducing agent that is lower on the series.
Limitations of the electrochemical series
If not at SLC, order of half-reactions may be different and predictions based on the electrochemical series may hence be unreliable.
Series gives no info about reaction rate (may not occur rapidly, noticebly).
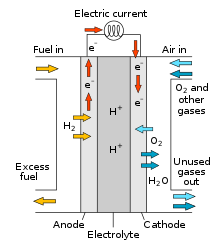
Fuel cells
A type of galvanic cell, converting chemical energy directly into electrical energy. The reactants (usually gases) are supplied continuously from an external source, allowing constant production of electrical energy.
fuel cells overcome the disadvantage with galvanic cells- they use a small amount of reactants, and must be discarded or recharged.
fuel cells don’t have the same energy losses as in fossil fuels, and the greenhouse gases impact. They are 40-60% efficient and can use the waste heat to make steam to operate a turbine, then making it 85%.
electrodes used in fuel cells allow direct contact between the gases and the electrolyte, but separate the gas compartments. they hae a high surface are and are porous to ensure high cell efficiency, often containing catalysts to increase rate of reaction.
high-cell efficiency. surface area includes the area within each pore. more surface are = rate reaction increase as there is the same flow rate of gas but more molecules are in contact with catalysts, to produce a greater current.
oxidation of a fuel, like hydrogen, happens at the anode, and the reduction of oxygen in the cathode. cleanly and efficiently generates electricity.
an electrolyte (solution) in a fuel cell carries ions from one electrode to another different electrolytes can be used infuel cells.
temp also increases rate of reaction, like catalysts.
Fuel cells and the hydrogen economy
fuel cels are important for a hydrogen economu, as hydrogen could become a major source of energy that replaces fossil fuels.
with hydrogen products are electricity, heat, water.
lasts as long as the fuel is supplied.
Expand on what fuel cells are classified by
The kind of electrolyte used.
Alkaline fuel cells. Like KOH OR NaOH. Makes it alkaline conditions.
each cell is about 1V.
Acidic fuel cells. phosphoric acid.

Advantages and disadvantages of fuel cells
ADVS
more efficient than the series of energy conversions in fossil fuel power stations (chemical, heat, mechanical, electrical)
producing just as much energy for half the amount in coal-fired power stations, so less fossil fuels and emissions
by-products of hydrogen are hear and water, no greenhouse gases
fuel cells generate electricity as long as fuel is supplied
can use a variety of fuels
electricity can be generate on-site and waste heat can be used to heat water for a hot-water system of provide heating.
DISADVS
require constant fuel supply
expensive requires extensive network of hydrogen filling stations before becoming widespread
hydrogen is mainly sourced from fossil fuels, with energy losses and greenhouse gas emissions
storage and safety of hydrogen fuel
some cells use toxic electrolytes and their electrode contain rare/expensive/harmful materials
Why does the pH of the electrolyte in a fuel cell initially increase and then becomes a constant value?
initial increase: cell begins to produce electricity with reduction at the cathode, under alkaline conditions, produces hydroxide ions. but then they migrate to anode and when this rate of production = rate of departing, the pH near electrode becomes constant value.
How will fuel cells play a key role in the transition from a dependence on fossil duels for energy to a hydrogen economy?
Burning fossil is the dominant source of greenhouse gas emissions. From a fuel cell, they are much less than if that fuel were burnt in a power station or internal combustion engine.
Change how hydrogen is produced.
Hydrocarbons will be replaced by hydrogen, fuel cells replace internal combustion engines. Most fuel cells use hydrogen, with the only product being water. ‘zero-emission device’ )water, heat, electricity)
How is hydrogen currently produced?
95% of hydrogen produced come from fossil fuels, through steam reforming.
Steam reacts with fossil fuels at high temp. with nickel catalyst. Hydrogen has lower energy content than original fuel, as energy is lost as waste heat in exothermic reaction
There are CO2 emissions. Cut they could be captured and stored at the site to not escape.
It’s a bridging solution until cost to produce renewables lowers.
Using electric energy (from renewable sources) to convert water to hydrogen (electrolysis, like with a renewable fuel cell). Collect biogas from landfill and convert the CH4 to H2 by steam reforming.
How to make renewable? All is possible when increasing global resolve, production, distribution, storage, safety. This requires massive expenditure on infrastructure (changes to pipelines, filling stations, improving H storage methods). Circular economy- optimise use, reuse.
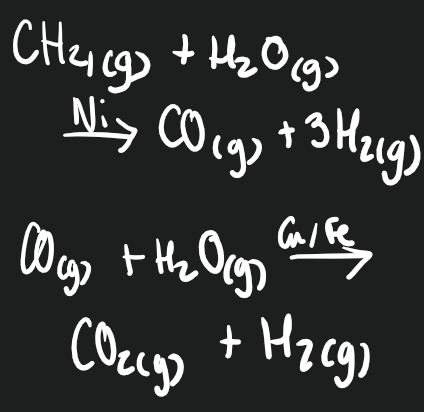
Compare hydrogen and fossil fuels
Hydrogen has very high energy content (143 kJ g-1) to fuels like petrol (44 kJ g-1). But it is hard to store as it is a gas at room temp. So store it as liquid/compressed (high pressure) but energy available per L is much less than for a liquid hydrocarbon fuel. Hydrogen would require a bigger fuel tank to have equal driving range. It’s a solution that still need developing: materials-based storage (uses materials that are solid or liquid to absorb or react with hydrogen and then release when needed)
There’s also a safety challenge- it’s highly flammable, even explosive, so needs some sensors to detect leaks, codes and standards. but it is not more dangerous than petrol.
Why are fuel cells more efficient for generating electricity than by combustion?
Fuel cells avoid the energy losses (one single transformation!)
How can we optimise efficiency of energy conversion and reduce fuel consumption and pollutant emissions?
Use alternative sources of energy- renewables.
Biofuels! (fuels from plants or organic waste). but, bioethanol and biodiesel come from crop using up land that would have been for food.
Though biomass (wood, manure) is inedible, it is low quality. That is like biogas, when burned, not a lot of energy is produced- less methane content.
Supplies need to be sustainable and minimise CO2 emissions.
So galvanic cells, like fuel cells.
Expand on how battery technology can be viewed in terms of two green chemistry principles.
Design for energy efficiency and use of renewable feedstocks.
Designing better fuel cells for renewable energy, energy storage, energy management, greenhouse gas reduction, safety, cost, lifetime and performance.
Avoid materials that pose environmental and humanitarian risks, like heavy metals nickel and cobalt. Cobalt in Central Africa is extracted in an unsafe and exploitative manner, with health risks. Platinum group metals (Pt, Pd, Rh) pose fewer risks.
Future: increased use of mobile devices, transport (non-renewable)
Feedstocks are the raw materials necessary needed to reproduce/manufacture other chemicals/materials. crude oil → gasoline. sun,wind,water, corn, hydrogen.
Use of renewable feedstocks… we need to balance the intermittent nature of the power generated (during windy weather, only during the day) with electrochemical energy generation and storage.
Applying microbial fuel cells.
MFC. A device that converts organic material to electric energy by action of microorganisms. Form a film on the anode’s surface and oxidises organic materials, usually to produce CO2, protons and electrons. Transfer electrons to cathode. Membrane separates anode and cathode, and balance charge by allowing movement of ions.
Works at room temp. uses low-grade waste materials (sediment, waste water, soil, ag waste), for water treatment/contaminant removal/low-power electricity.
35% efficient