Lecture #10 | Targets of Mutations: Oncogenes & Tumor Suppressor Genes Identification of the first Oncogene (Src)
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
Oncogene
A gene whose presence in certain forms and/or overactivity can stimulate the development of cancer
Tumor Suppressor Gene
A gene whose absence can lead to cancer
Do most mutations lead to cancer?
No, only 1% of the human genome codes for proteins (exons)
most mutations do not impact protein function and do not lead to cancer
What gene mutations can lead to cancer?
Mutations in oncogenes and tumor suppressor genes
Transformed phenotype
exhibits one or more properties of a cancer cell
Characteristics of normal cells
Require growth factors
Exhibit density-dependent inhibition of growth
Anchorage dependence
Want to have a surface to attach to
Finite proliferative life span
Do not endlessly grow
Adhesiveness
Characteristics of transformed cells
Require fewer, if any, growth factors
Do not exhibit density-dependent inhibition of growth
Anchorage independence
Infinite proliferative life span
Lack of adhesiveness → morphology
Form tumors in vivo
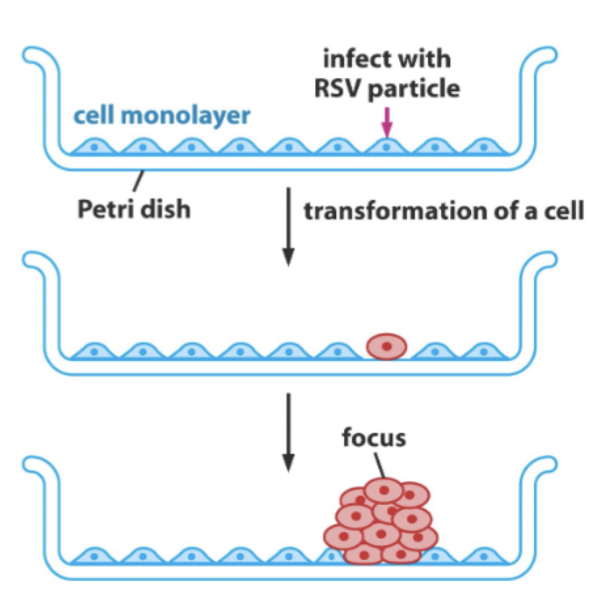
Details on the density-dependent inhibition of growth
In normal cells, they form in a monolayer on a Petri dish
In transformed cells that lack this density-dependent growth, they form a foci
Does not care that there are a lot of cells around
Can expand and impact normal cells
RSV causes the transformation of the cell
Growing on top
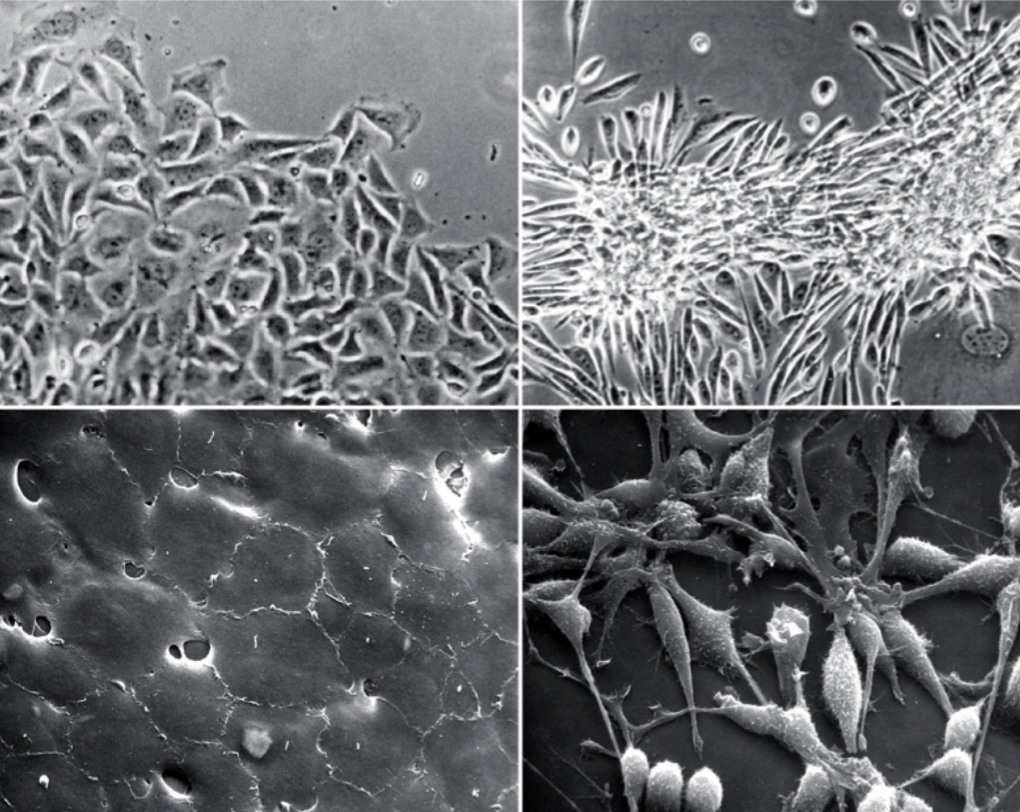
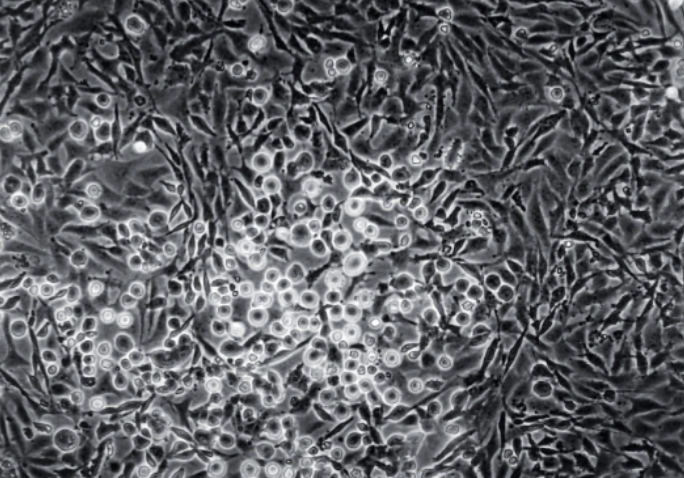
Which is a normal cell and transformed?
On the left → normal; aware of the space
On the right → transformed; growing on top of each other
Anchorage independence experimentation for transformed cell
Uses a nude mouse model
lack a thymus → immune compromise
Lacks the immune response to fight foreign cells
develops tumors fast
Lack hair
Allows for easy visualization of tumors
Ways that a transformed phenotype can arise
Activation of a proto-oncogene in to an oncogene
Mutation or deletion of a tumor suppressor gene
Proto-oncogene
A normal gene that can be changed into an oncogene
Proto-oncogene → mutation → oncogene
An activating mutation in one copy of a proto-oncogene can lead to the transformed phenotype.
When does a mutation of a tumor suppressor gene result in cancer?
Both copies of a tumor suppressor gene must be mutated for cells to be transformed
Normal function of oncogenes and tumor suppressor genes
They regular the cell cycle
proto-oncogenes result in proliferation (division, growth) signals
Tumor suppressors stop growth
Cell cycle
G1 (cell growth)
Oncogenes
Tumor suppressor genes
S (synthesis)
Tumor suppressor genes
DNA repair genes
G2
Mitosis
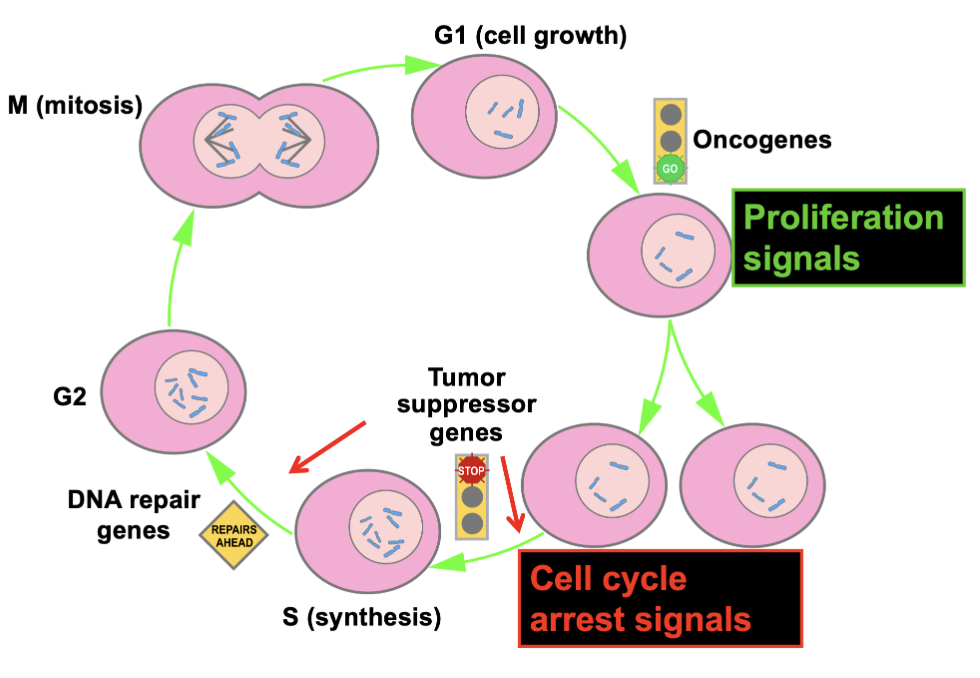
Oncogenes in cell cycle
Act like the gas pedal
Activating mutations in oncogenes tend to be dominant
You need to mutate only one copy to get transformation.
Proto-oncogene to oncogene 1st mutation accelerated cell division
Activating mutations in oncogenes are like a stuck accelerator - the gas pedal is always on!
Tumor suppressor genes in cell cycle
Are like brakes
Inactivating mutations in tumor suppressor genes tend to be recessive -- both copies must be altered.
1st mutations: cell in susceptible to cancer
2nd mutation or loss: leads to cancer
No brakes at all
Normal function of proto-oncogenes
Activate the signal transduction pathway and push the cell cycle forward
stimulates cell proliferation
What do tumor suppressors normally do?
Act as brakes on the signal transduction pathway and cell cycle
mutations cause lose of ability to inhibit cell proliferation if needed
Signal transduction pathway
Normal cells need a signal from a growth factor (a peptide) to enter the cell cycle.
Growth factors bind receptors on the surface of the cell.
The receptor becomes active, often via dimerization, & sends a signal -- phosphorylation.
This starts a cascade of short-lived phosphorylation events.
The ultimate targets are transcription factors (TF) in the nucleus and other proteins that regulate the cell cycle.
Proto-oncogenes can be anywhere in the ST pathway: Growth factor, receptor, kinase (enzyme that can add a phosphate), TF, cyclins.
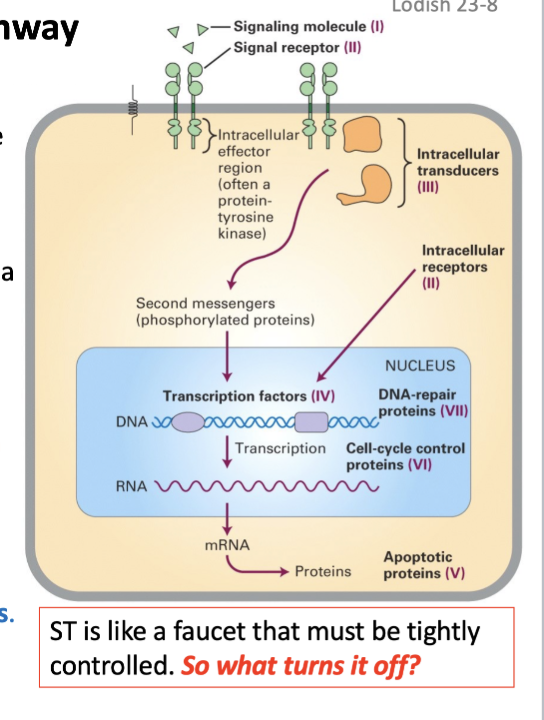
Role of protein kinase
Kinase adds a phosphate (PO_4) to Ser (S), Thr (T) and Tyr (Y).
very specific, PO4’g only specific aa consensus
sequences
>500 kinases in the human kinome
Many more Ser/Thr kinases than Tyr kinases
STKs are more ancient and widespread, playing crucial roles in fundamental cellular processes, while TKs are a more recent innovation, often involved in more specialized signaling pathway
Role of protein phosphatase
Removes the (PO_4)
non-specific, acting on a wide range of phosphoproteins.
<200 protein phosphatase genes in human kinome
Retroviruses
RNA Tumor Viruses
Retroviruses can productively infect only proliferating cells.
HIV (aka AIDS) is a classical retrovirus
The viral RNA genome is reverse transcribed into proviral DNA which integrates randomly into the host genome.
How is RNA reverse transcribed into proviral DNA
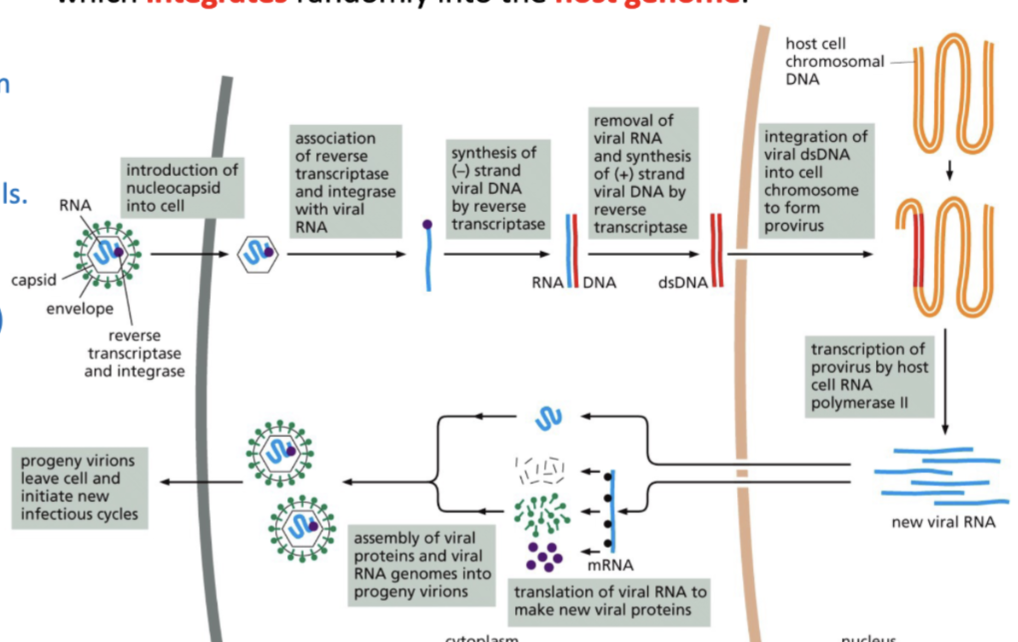
RNA has reverse transcriptase and integrate
after infection, these help in synthesis of negative DNA and then then RNA is removed
+ strand of viral DNA is synthesized by reverse transcriptase
this integrates into viral dsDNA into cell chromosome to form provirus
Can infect only proliferating cells
What was the first demonstration that a virus can cause cancer?
In 1911 Sir Francis Peyton Rous experimented with chickens with sarcomas.
Cancer of the breast muscle
Extracted the tumors, grinder it up, processed in a filtrate
Why filtered? Make sure to get rid of other infectious agents like bacteria
injected into another chicken and saw sarcomas in 1-2 weeks!
Much later, the tumor extracts were found to produce virus that transformed cells.
50 yrs later, Rous Sarcoma Virus (RSV) was isolated -- Rous won Nobel prize 1966
Rous Sarcoma Virus
Has spike proteins
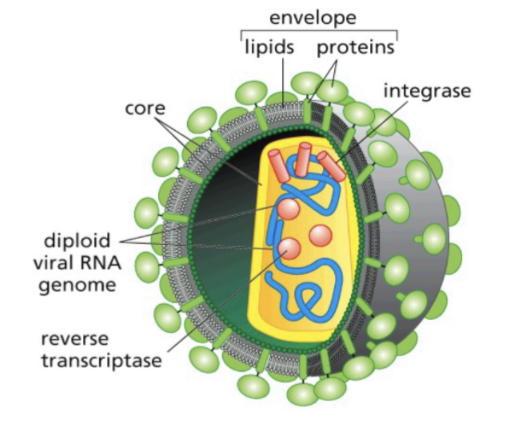
Attached on cell surface of the host
Core made up of lipids and proteins
SsDNA has a reverse transcriptase and integrase
Reverse synthesis
Helps with integration
Not all retroviruses are infections
Basic structure of viral RNA genome
gag which codes for core proteins
pol which codes for reverse transcriptase and integrate
and env which codes for protein
Not always infections
Acutely transforming virus (e.g., RSV):
In vivo: tumors in 1-2 weeks
In vitro: infect (& replicate) and transform cells
Able to produce tumors and transform cells in Petri dish
Virus can infect and transform the cell
Non acutely transforming virus (e.g. ALV):
In vitro: infect (& replicate) but not transform cells
Infect but not transform cells
In vivo: minimal tumors in 2-3 months
Can infect but not transform the cell
What is the difference between transforming and non transforming virus?
V-onc
V-onc
Viral oncogene
How does ALV → RSV
Capture c-src (cellular carcoma)
Found in the host cell chromosomal DNA
ALV infected a cell and accidentally integrated next to c-src
So c-src + provirus → fused ALV-src RNA transcript
Packaged into capsid carrying src sequences
So ability to transforms cells comes from host cell chromosome
How are v-src and c-src different?
V-src has no introns, mutated → always activated
How did they identify the cellular version of v-src
Southern blot analysis using a radio labeled v-Src probe
They found a Src gene not just in chickens but in every other higher eukaryote they examined, including humans.