CAR week 8: BBB, circulation, infection and septic foal
1/82
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
83 Terms
What are 3 ways microbial pathogens can damage the nervous system?
Invasion and replication in the tissues
inducing an immune response
releasing toxins
How can microbial pathogens damage the nervous system by invasion and replication in the tissues?
direct invasion of peripheral tissues
from adjacent structures such as the meninges
from blood Haematogenous
How can microbial pathogens damage the nervous system by inducing an immune response
inflammation of CNS
damage cause by local inflammation to the CNS
auto-immune response.
How can microbial pathogens damage the nervous system by releasing toxins?
blocking signals
damaging specific cells
What are the 3 ways microbes can spread and access the CNS?
neurotropic
neural abscess
haematogenous
What do these mean:
neurotropic
neural abscess
haematogenous
from peripheral nerves: nerve to nerve
neural abscess
via blood
What words can be used to describe viruses and bacteria that spread via blood?
bacteraemia
viraemia
Why is the CNS at such high risk of haematogenous spread?
for every neurone there’s a blood vessel
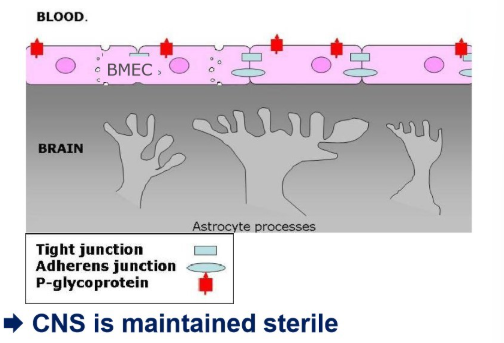
Outline 4 essential features of the BBB
endothelial cells (BMECs) form a tight barrier b/w blood and CNS-tissue
tight junctions stop paracellular flux
pinocytosis allows transfer of material from blood to brain
P-glycoprotein pumps and actively ejects undesired substances
What are 3 methods of breaching the BBB>
transcellular
paracellular
intracellular within leucocytes
Outline transcellular invasion:
what does it mean
active or passive?
examples
pathogens bind host cells and invade through the cell
passive or active
bacterial: Streptococci, Listeria sp.
fungal: Candida (yeast), Cryptococcus (dia-morphic fungus)
Outline paracellular invasion:
what must happen
what can make it easier?
examples
tight junctions must be dramatically changed/new routes open
increased pinocytic activity → trans-endothelial channel formation/tight junction can be broken
viral: Nipah virus
Bacterial: Borrelia burgodorferi
Outline intracellular infection from within leucocytes:
what does it require
how does it spread
examples
primary infection
where the virus migrates to - may be ‘hidden’ to antibodies once established
viral: SIV/HIV, canine distemper
What is central to infection of microbes crossing the BBB?
expression of virulence factors
How can viruses cross the BBB?
they have host cell binding proteins that bind receptors on endothelial cells, essential for infection to progress
How can bacteria cross the BBB?
adhesion molecules
common surface proteins/outer membrane proteins (OMPs) that increase adhesion of bacteria to the cell
binding may trigger specific invasion mechanisms
once inside the cell, bacteria require virulence factors to survive and acquire nutrients in intracellular environment
how can inflammation damage the CNS?
accumulation of leucocytes → causing pressure to increase in cell numbers
clinical signs reflect the damage caused by specific areas of the CNS
pathogens ‘may’ cause cellular pathology
what may clinical signs of CNS damage include?
depression
pyrexia
cervical pain
hyperaesthesia
photophobia
generalised rigidity
seizures
paralysis local and general
ataxia
papilloedema
possible ophthalmic inflammation
systemic signs: septic shock and brachycardia
Outline normal CSF:
colour
total white cell count
total red blood cell count
cytology
total proteins
specific gravity
colourless
<6 cells per ul
0
few monocytes, lymphocytes and rarely neutrophils
cisternal <0.3g/l, lumbar: <0.45g/l
1.007
With CNS infection what variables can we test in the CSF
glucose
specific gravity
immunology
microbiology
enzyme analysis for cell breakdown
How will glucose and specific gravity change with infection on assessing CSF?
glucose decreases
specific gravity increases
what 2 things do we test in immunology when testing CSF fluid for infection?
serology
cytology
how is the CSF maintained?
it’s maintained as sterile
Name 3 diseases and the 3 different infection routes they take
canine distemper - viraemia (haematogenous/broad infection)
listeriosis - neurotropic spread-bacterial
rabies - neurotropic spread-viral
Outline CDV:
what is it
how does it develop
what kind of microorganism?
who does it infect
infectious?
how does it spread
how does the infection spread within the animal
what organs does it affect
what do we see in the acute phase
what do we see in the post-acute phase?
canine distemper virus
paramyxoviridae → morbillivirus → canine distemper virus
large RNA virus, single stranded. Enveloped - inactivated by most detergents and soap. narrow host range
infects mainly mammals and birds
highly infectious of dogs and other carnivores
urine, close contact or aerosols
URT → bronchial LN and tonsils → blood stream → various tissue organ systems → meningeal macrophages → neurones
CDV is pantropic - infection in many organ systems as receptors are common on many cells
encephalitis due to virus infection of neurones
encephalitis due to inflammatory response incited by virus
Outline CDV microbe:
what kind of virus
how does it attach to cells
how does it replicate
pleomorphic enveloped virus, negative sense single stranded RNA
F-protein
in cytoplasm buds as it’s enveloped
What is the relevance of CDV being an enveloped virus?
relatively labile
sensitive to heat, desiccation, lipid solvents, non-ionic detergents and disinfectants
isolation of affected animals and disinfection can control outbreaks
vaccines: H-protein can be used to induce neutralising antibodies
Outline CDV spread of infection:
where does it replicate
where does it spread to
what does replication lead to
what other sites does it affect
what neuronal tissue does it affect
how can it spread neurone to neurone
URT
tonsils and bronchial lymph nodes
lymphocytolysis/leukopenia → viraemia
GIT, urinary, CNS tissue
meningeal macrophages, astrocytes and neurones
without cytolysis
How do we diagnose CDV?
lateral flow test detecting CDV antigen
PCR
Outline use of lateral flow tests for detecting CDV antigen:
what do we need to use for it
what is recommended
what kind of test is it?
ocular and nasal secretioins
confirmation by PCR
pen site test
Outline PCR for CDV diagnosis:
how long does it take
what is it a measure of
what does it enable the test to do
when will we detect more?
1-3 working days
quantitative measure of viral load
discriminate vaccine interference from infection with a wild-type of strain
during infection rather than because of recent - enabling us to determine whether the animal is actively infected
Outline listeriosis:
general features of the bacterium
is it zoonotic?
source?
main clinical manifestation?
gram +ve, rod, facultative anaerobe, small haemolytic colonies
yes
in cattle - poor silage, generally, source eating contaminated food
sepsis and meningitis
How is meningitis often complicated with listeriosis?
it’s often complicated by encephalitis - an unusual pathology for bacterial infections
Outline Listeria monocytogenes:
how do they affect cells
how do they move?
what kind of infection method to the CNS
attach to and enter cells
move wtihin and b/w by nucleating actin
neurotropic: nerve cell to nerve cell
Outline listeriosis in ruminants:
where is it found
where can they replicate
when do we see outbreaks
how may it present
what’s a source of susceptibility
environment
poor quality silage, pH above 5.5
seasonally
encephalitis, abortion, septicaemia or endophthalmitis
decreased cell-mediated immunity associated with advanced pregnancy
Outline listeriosis in ruminants:
clinical signs and incubation period
treatment
prevention
14-40day incubation period, dullness, circling, tilting of head facial paralysis, unilateral facial paralysis → drooling and dropping of eyelids and ears
in early stages with antibiotics
don’t feed poor silage, ensure feed method reduced ocular contact and vaccines don’t work
Outline rabies:
what causes rabies
what kind of virus
shape
where is it found
how is it transmitted
incubation period
prognosis
relevance globally?
what is indistinguishable from it?
Rhadoviridae → lyssavirus → rabies virus
enveloped RNA virus
rod shaped
saliva
biting carnivores
14-90 days, b/w bite and development of signs of neurological involvement
fatal
notifiable disease
a number of neurotropic lyssaviruses that are closely related to rabies
Outline the stages of rabies spread to nervous tissue
bite
virus replicated in muscle
high titre → reach sensory/motor nerve ends
binds ACh receptor
virus enters into distal nerve and second stage of infection begins
neuronal infection with centripetal passive movement within axons
delivery of virus to CNS initially spinal cord
reaches limbic system in the brain, replicates extensively → swelling → pain and behavioural changes
spread continues clinical replication in cortex, pathology low
centrifugal spread to periphery 0> organs → salivary glands
what is meant by ‘furious rabies’
when the virus reaches the limbic system of the brain and replicates extensively
this leads to swelling of brain tissue
pain and behavioural changes
What is meant by ‘dumb’ rabies
when the spread of the virus continues clinical replication in the cortex
cell pathology is low but cells all contain the virus
How do we diagnose rabies?
post mortem brain material:
fluorescent antibody test (FAT) for viral antigen
How do we treat rabies:
confirmed cases
suspect cases
mandatory euthanasia
isolation
what do we have to do for rabies cases where there’s a potential risk of a person being bitten/scratched by the infected animal/
VI (veterinary inspector) requires destruction of the animal in order to confirm or rule out diagnosis of rabies by post mortem
What is the rabies vaccination?
inactivated vaccine/recombinant vaccine
not required in UK for domestic species - only if travelling abroad, to obtain Animal Health certificate
How does the vaccine for humans for rabies differ to the one for animals?
active vaccination for humans - needs to be administered rapidly, inactive once neurological signs present
inactivated vaccine/recombinant vaccine
What are the 2 ways toxins become present in the body?
ingestion
infection
How are toxins produced via ingestion?
by fungi, plants, microorganisms and ingested via contaminated pasture and feed
How are toxins found in the CNS by infection?
toxins are produced by colonisation of young animals by toxin producing bacteria normally excluded from the intestine
can be produced during infections
what are 3 examples of toxin producing bacteria?
tetanus - Clostridium tetani
Botulism - Clostridium botulinum
oedema disease
What are examples of toxins produced by fungi, and where?
rye grass staggers (new zealand) - sporadically in UK with imported rye grass
on pasture
What plants produce toxins (relevant)
algae
give 3 examples of non-microbial toxins
lead
arsenic
organophosphates
Outline clostridia:
describe the morphology
type of respiration
how can we kill the cells
what about the spores?
relatively large, gram +ve, rods, form endospores
strict anaerobe
vegetative cells are killed by O2 exposure
spores can survive long periods of exposure to air
what are 4 kinds of toxins?
neurotoxic
histotoxic
enteropathogenic
A-typical
what species of bacteria produce neurotoxic toxins, what kind of toxins are they
C.tetani
C.botulinum (types A-G)
potent AB neurotoxins
what are toxins
proteins that area activated by proteolytic cleavage
Outline the tetanus toxin:
type
what does it cause and how
1 antigenic type
synaptic inhibition → muscular spasms
Outline botulinum toxin:
types
what does it lead to and how?
7 antigenic types
inhibits neuromuscular transmission → flaccid paralysis
Outline tetanus:
what does it cause and how
how is it produced
what is a clinical sign
what 2 kinds are there?
continuous stimulaiton by stopping inhibitory transmitter binding at synapse
organisms replicating locally in tissues/wounds → toxicoinfectious
spastic paralysis: muscular spasms
descending and ascending
Outline ascending tetanus:
what is the pathway of the toxin and what does it lead to?
toxin disseminated in bloodstream
remote areas
enters CNS at many levels
generalised tetanus, often beginning cranially (horses)
Outline ascending tetanus:
where does the toxin go
what does it bind to
what does it prevent
what does it lead to
peripheral nerves
specific gangliosides on motor nerve terminals
suppresses release of afferent inhibitory neurotransmitters
spastic paralysis and characteristic spasms → carnivores
How do we diagnose tetanus
clinical evaluation
toxin presence confirmed by PCR assay of bacterial DNA from wound tissue
How do we treat tetanus:
what’s available for horses
what should we do
vaccine and cattle
early intervention: wound cleaning, boosting immunity, parenteral antitoxin administration and muscle relaxants
not vaccinated animal → antitoxin prophylactically
supportive therapy: monitor for normal parameters to return
antitoxins neutralise circulating toxin are only effective before the toxin has entered the nerve terminals
recovery is by axonal sprouting and re-innervation (slow (up to 3 months))
Outline botulism:
where does the toxin move to and what does it prevent
where is it produced
sources?
where does it enter in the body
what does it cause
nerve terminal and inhibition of ACh release
decaying organic matter
carrion/colonised, feed rotting hay/silage → intoxication
absorbed into the bloodstream and distributed around the body
flaccid paralysis
How do we treat botulism?
supportive therapy
antibiotics aren’t activated as they have limited effect on bound toxins
euthanasia
why are ruminants with botulism usually euthanised?
clinical signs won’t resolve until neurotoxin has decayed
this takes weeks/months
impractical to attempt nursing for this period of time and consideration of animal welfare needs to be given
55 hr-old Thoroughbred colt has been rushed into your hospital with weakness and sudden onset respiratory distress. The foal was born at 335 days gestation, and was the first foal from this dam. The foaling was attended and the delivery process seemed normal, including expulsion of foetal membranes. The foal stood and sucked within 2 hrs of birth, passing normal-looking meconium, and behaved normally during the first 48 hrs of life.
This morning (the 3rd day post-partum) the foal was found recumbent, dehydrated and breathing rapidly. It was immediately transported to your hospital.
Physical examination:
On arrival the foal was weak and unable to stand without assistance
Body weight: 54 kg
Dehydration was estimated at 5-7% of body weight
Mucous membranes: dark pink
Capillary refill time: 2s
Pulse: weak and regular
Blood pressure: systolic 75mm Hg, diastolic 45mm Hg, mean 55 mm Hg (normal: 100/60, mean 80)
Rectal temperature: 103ºF/39.3ºC (should be 37.5-38.6)
Heart rate: 70-80 bpm.
Resp rate: 60 breaths per minute, weak and shallow
Thoracic auscultation: bilateral wheezes and crackles, more pronounced over the ventral lung field
For a septic foal what should our emergency treatment plan be?
oxygen (preferably humidified if dehydrated) - face mask/nasal insufflation (6-10l/min)
blankets and heat lamps to keep foal warm
IV fluid in septicaemic foal to combat cardiovascular collapse, avoid too vigorous IVFT (riskk of pulmonary oedema)
sternal recumbency
55 hr-old Thoroughbred colt has been rushed into your hospital with weakness and sudden onset respiratory distress. The foal was born at 335 days gestation, and was the first foal from this dam. The foaling was attended and the delivery process seemed normal, including expulsion of foetal membranes. The foal stood and sucked within 2 hrs of birth, passing normal-looking meconium, and behaved normally during the first 48 hrs of life.
This morning (the 3rd day post-partum) the foal was found recumbent, dehydrated and breathing rapidly. It was immediately transported to your hospital.
Physical examination:
On arrival the foal was weak and unable to stand without assistance
Body weight: 54 kg
Dehydration was estimated at 5-7% of body weight
Mucous membranes: dark pink
Capillary refill time: 2s
Pulse: weak and regular
Blood pressure: systolic 75mm Hg, diastolic 45mm Hg, mean 55 mm Hg (normal: 100/60, mean 80)
Rectal temperature: 103ºF/39.3ºC (should be 37.5-38.6)
Heart rate: 70-80 bpm.
Resp rate: 60 breaths per minute, weak and shallow
Thoracic auscultation: bilateral wheezes and crackles, more pronounced over the ventral lung field
What is abnormal?
rectal temperature
pulse
heart rate
dehydration
resp rate
thoacic auscultation
blood pressure
unable to stand without assistance
what diagnostic tests can we perform on a septic foal
biochemistry
abdominal ultrasound
blood culture for bacterial infection
BGA
haematology
maternal antibody transfer test
thoracic radiography
thoracic ultrasound
urine sample
why do we perform abdominal ultrasound for a septic foal?
check for potential sources of bacteraemia
Why do we perform biochemistry in a septic foal
serum/plasma protein level to assess hydration
hypoglycaemia is common in septicaemia
plasma levels elevate in septic shock
creatinine and urea values are often raised
foals with concurrent enteritis may develop severe electrolyte imbalances
why do we test for maternal antibody transfer in foals with suspected septic shock?
failure of passive transfer is the most common predisposing factor to neonatal infection
why do we perform thoracic radiography on foals with suspected septic shock
radiology appearance of pneumonia may indicate aetiology and pathogenesis of the condition
why do we perform:
thoracic ultrasound
urine sample
in a foal with suspectiv septic shock
demonstration of pleural fluid or consolidation of peripheral lung tissue - greatest value in conjunction with thoracic radiography
assess kidney function
What will the haematology of a foal with pneumonia show?
leucocytosis/leucopenia
neutrophilia
increased no. band neutrophils and toxic changes
hypoglycaemia
plasma lactate levels elevate
creatinine and urea raised
lymphopenia can occur in overwhelming infections
plasma fibrinogen levels are elevated
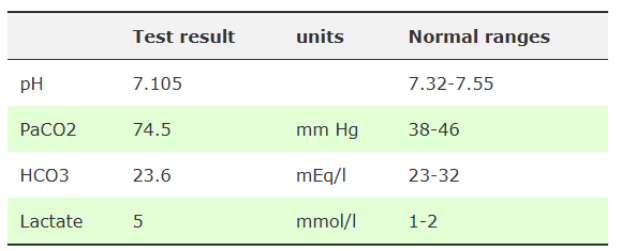
What do these findings show?
severe respiratory acidosis
mild metabolic acidosis
poor O2 perfusion of tissues
What do these show?
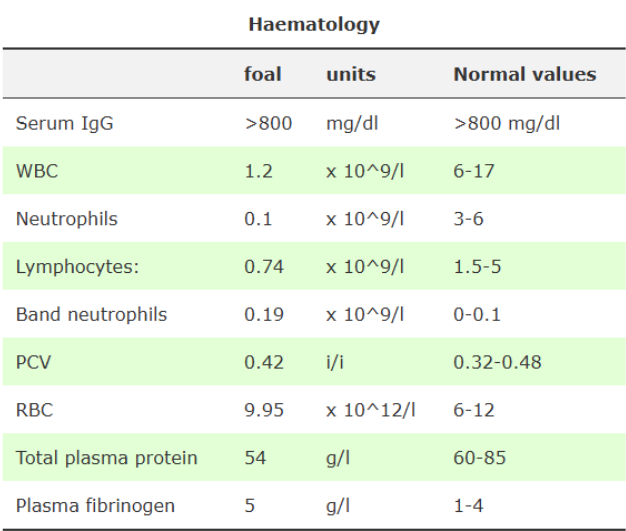
IgG levels >800: successful passive transfer of maternal antibodies has occurred
severe neutropenia: severe infection (usually opposite with bacterial pneumonia unless severe) - poor prognosis
lymphopaenia: stress associated with overwhelming infection
degenerative left shift: confirm presence of neonatal infection
what are the mortality rates for foals developing severe sepsis and septic shock?
severe sepsis: 52%
septic shock: 67%
How do we treat sepsis and bacterial pneumonia
Management: mare stabled, supportive treatment and antibiotic therapy
What supportive treatment can we provide for a foal with sepsis and bacterial pneumonia?
padding
frequent turning of recumbent foal
O2 administration
intravenous fluids preventing cardiovascular collapse
nutritional support through nasogastric tube
mare’s milk/substitude and/or parenteral nutrition
What do these recognise:
TLR2
TLR4
TLR5
TLR9
cell wall components of gram +ve bacteria: can form heterodimers with TLR1 or TLR6
LPS of gram -ve bacteria
flagellum
bacterial DNA through CpG molecules
Outline the process of bacterial infection to MODS
PAMPs are recognised by host receptors especially TLRs
adapter molecules
signalling network
production of inflammatory mediators
local inflammatory infection
bacteraemia/bacterial products in blood stream
systemic inflammatory response
sepsis/SIRS
sever sepsis
MODS: multiorgan dysfunction syndrome