APES UNIT 7—ATMOSPHERIC POLLUTION
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
7.1—Introduction to Air Pollutants
I. Describe the power given to the EPA by the Clean Air Act
II. Explain why CO2 is typically not considered an air pollutant
III. Primary vs secondary air pollutant
I. Ability to set, monitor, and enforce acceptable limits for 6 criteria air pollutants
II. CO2 does not decrease air quality from a human health standpoint; does not directly cause health consequences in the air we breathe
III. Primary—emitted directly from a source (ie. SO2 or PM coming from a coal-fired power plant) vs Secondary—when primary undergoes some sort of transformation in the atmosphere once exposed to air, water, and oxygen (ie. sulfuric acid from the reaction of sulfur dioxide and water)
SOx Sources and Effects
Emited by coal combustion for electricity production, volcanic eruption
Causes acid precipitation, respiratory damage
NOx Sources and Effects
Caused from FF combustion from gas, biomass combustion, forest fires
Causes acid precipitation, respiratory irritation, photochemical smog from ozone
CO Sources and Effects
Caused from incomplete FF and biomass combustion
Causes asphyxiation (suffocation)
PM Sources and Effects
Caused by FF and biomass combustion, construction, agriculture, industrial production
Causes respiratory irritation, reduced visibility due to thickening smog
Tropospheric O3 Sources and Effects
Caused by the reaction of NO2, sunlight, and O2
Causes respiratory irritation, contributes to photochemical smog, plant tissue damage
Lead Sources and Effects
Caused by coal combustion, ore metal processing plants, and lead smelting factories
Causes damage to the central nervous system as a neurotoxicant
7.2—Photochemical Smog
I. Identify precursors/reactant of photochemical smog formation
II. Identify the environmental conditions that increase PS formation
III. Describe how tropospheric ozone forms
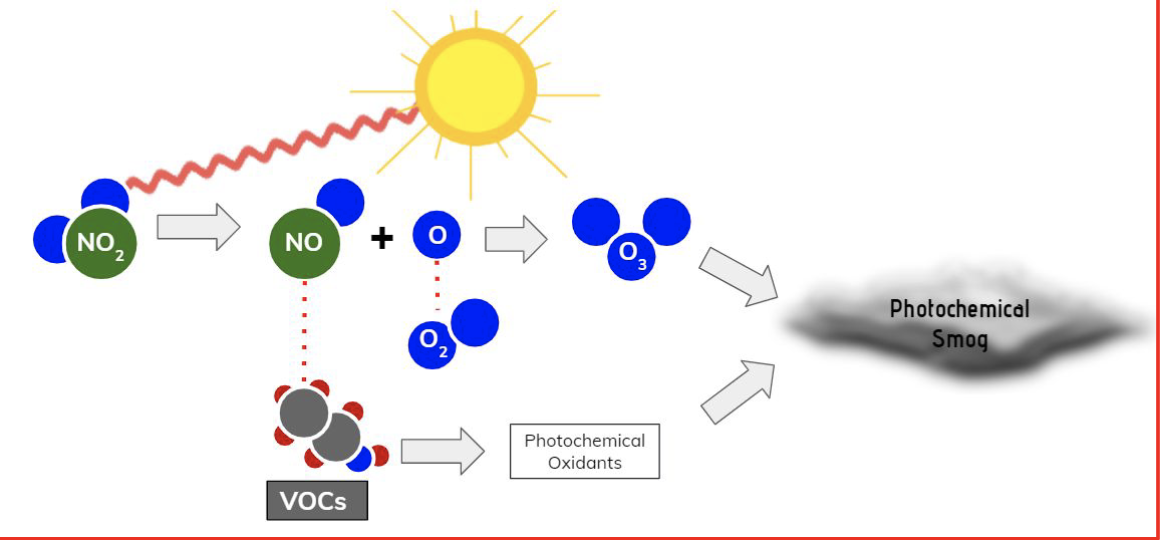
I. NO2, VOCs (volatile organic compounds), O3
II. sunlight and heat
III. energy from the sun causes NO2 to split into NO and free oxygen atm; the free oxygen item then combines w/ O2 in the troposphere to form O3
7.2—Photochemical Smog
I. Explain why tropospheric ozone levels normally decline overnight
II. Describe the role of VOCs in preventing tropospheric ozone levels from decreasing
III. Describe an environmental and human health impact of PS
I. as the sun goes down, it no longer drives the breakdown of NO2 into NO and O. Ozone reacts with the free NO in the atmosphere to re-form NO2 and O2.
II. when present in the atm., VOCs bind to NO, preventing O3 from reacting with free NO in the atm and reverting back to NO2 and O2
III. can cause respiratory irritation, worsening of asthma, eye irritation, reduced photosynthesis rate and damage to plant tissue (stomata)
formation of photochemical smog
7.3—Thermal Inversion
I. Identify normal temp-altitude relationship in the troposphere and explain why this relationship exists
II. Describe how this relationship normally helps disperse air pollutants near Earth’s surface
III. Explain how conditions of thermal inversion impact normal T-alt. relationship in the troposphere
I. As altitude increases, T decreases (in the troposphere) b/c air is under less atm. pressure; also further away from the infrared radiation (heat) released from earth’s surface
II. air near the surface is warmed b/c Earth’s surface releases heat due to incoming solar radiation it receives; air rises, causing pollutants near ground to be carried up and way from Earth’s surface
III. during a thermal inversion, a colder/more dense air mass is trapped beneath a warmer, mid-latitude air mass, causing a reversal of the normal T-alt. relationship near the Earth’s surface
7.3—Thermal Inversion
IV. Describe how a thermal inversion impacts ground-level air pollutant levels
V. Explain the urban heat island effect
IV. prevent the upward movement of air away from Earth’s surface, concentrating/trapping air pollutants near ground level
V. urban areas w/ higher T due to greater concentration of dark/low albedo surfaces that absorb sunlight and release infrared radiation (heat); also have less vegetation, which means less evapotranspiration cooling
7.4—Atm. CO2 and Particulates
I. Identify three natural sources of CO2
II. Explain how decomposition can produce both CO2 and CH4
I. cellular respiration, aerobic decomposition, forest fires, volcanic eruptions
II. in oxygen-rich conditions, aerobic decomposition produces CO2; in oxygen-poor conditions, anaerobic decomposition produces CH4
7.4—Atm. CO2 and Particulates
III. Describe the relationship b/w PM particle size and human health
IV. Three sources of natural PM
III. the smaller the particle, the deeper into the respiratory tract it can penetrate, causing more damage; larger particles typically more easily filtered out by respiratory structures less likely to penetrate more deeply
IV. forest fires, wind-borne soil/dust, pollen, ash, sea salt, mold
7.5—Indoor Air Pollutants
I. Describe the major source of indoor air pollution in developing countries and identify two indoor air pollutants released by this source
II. Human health impacts of indoor biomass combustion
III. Solution to reduce the impacts of indoor air pollutants in developing countries
I. indoor biomass combustion for heating/cooking releases indoor air pollutants such as CO, PM, VOCs, and NO2
II. headaches, eye damage, respiratory irritation, lung damage, worsening of asthma
III. better ventilation in homes (chimney, open windows, fans) to disperse pollutants, cooking outdoors, better access to stoves for cooking
7.6—Reduction of Air Pollutants
I. Individual and gov’t scale method for reducing air pollutants
II. Identify air pollutant that vapor-recovery fuel nozzles reduce
III. Describe how catalytic converters reduce air pollutants emitted from vehicles
IV. Explain how wet and dry scrubbers reduce air pollutants from factory/power plant emissions
V. Describe an add’l method for reducing PM from power plant or industrial emissions
I. solo: drive less and use public transit instead; purchase and use an electric vehicle; gov’t: raise CAFE vehicle standards, subsidies/tax credits for non pollution emitting power plants, rebates/tax credits for people purchasing electric vehicles, stronger regulation enforcement
II. VOCs/hydrocarbons/benzene
III. metals inside catalytic converter like palladium or platinum convert CO, hydrocarbons, and NOx into CO2, N2, O2, and H2O
IV. air stream from combustion rxns or other industrial processes passes thru a column that traps PM, SOx,, NOx, VOCs. Wet scrubbers use a mit sprayer and mist eliminator screen at the top of the column to trap pollutants in droplets of water that fall into a sludge collector at the bottom of the column; dry scrubbers use a chemical agent (e.g. calcium oxide) to bind to air pollutants like SO2 and trap them in a column
V. baghouse filters are cloth/fabric bags that a waste air stream passes through, trapping PM; electrostatic precipitators use an electrode to give all the particles in a waste air stream a negative charge so that they then stick to the positively charged metal plates as they pass through the device
7.7—Acid Deposition
I. Identify primary pollutants that lead to acid deposition and identify a major source of each
II. Describe the transformation these primary pollutants undergo to become secondary pollutants contributing to acid deposition
III. Describe how the disassociation of nitric acid and sulfuric acid in water results in acid deposition
IV. Environmental consequence of acid deposition
I. sulfur dioxide (coal power plants), nitrogen dioxide (coal power plants, vehicle emissions)
II. SO2 and NO2 react w/ water and oxygen in the atm., forming nitric acid and sulfuric acid and contributing to acid deposition
III. sulfuric and nitric acid disassociate into sulfate and nitrate ions and H+ ions in the presence of water; increase H+ ion concentration makes for a more acidic precipitation
IV. soil acidification lading to aluminum toxicity/plant or soil organism mortality; surface water acidification leads to aluminum toxicity and mortality
7.8—Acid Deposition
I. Sources of noise pollution
II. Effects of noise pollution
I. traffic, construction, lawn mowers, ships/tankers, seismic surveying ships. industrial processes
II. difficulty communicating, migratory disruption, increased HR, headaches
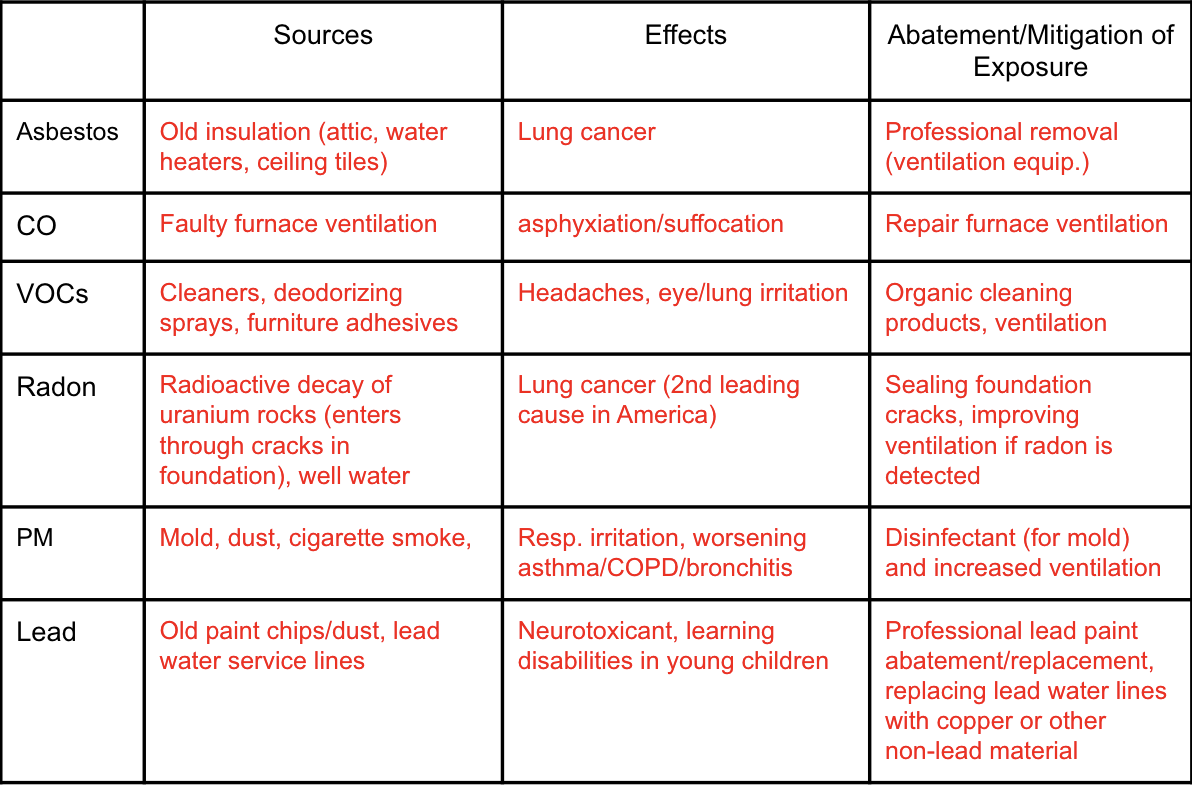
Sources, effects, mitigation of the following indoor air pollutants: asbestos, CO, VOCs, Radon, PM, and Lead