Organic Chemistry Final
1/80
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
81 Terms
H-Br
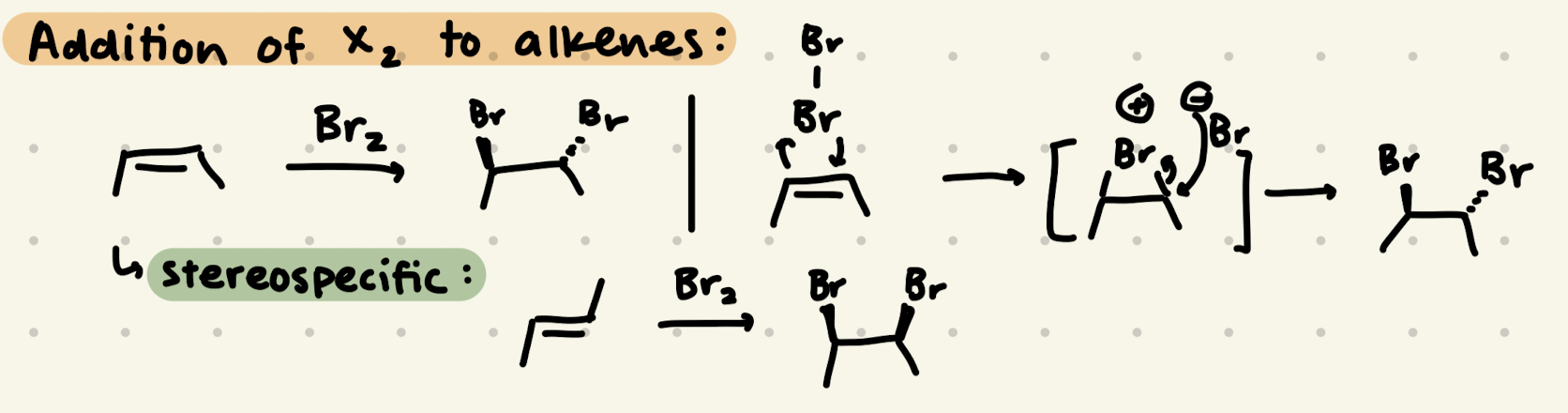
H-X addition to alkenes:
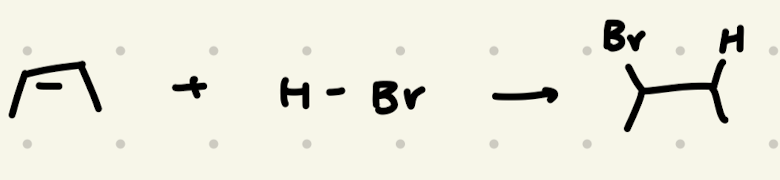
1) adds to resonance stabilized carbocation first
2) adds to more stable carbocation
3) look out for EWG’s (they’re less stable)
H2SO4, H2O
Addition of H2O:
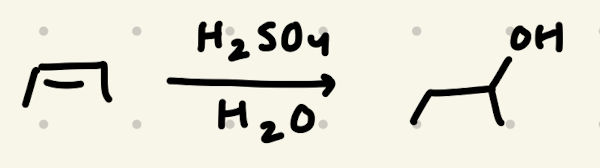
1) Adds to resonance stabilized carbocation
2) adds to more stable carbocation
H2SO4, R-OH
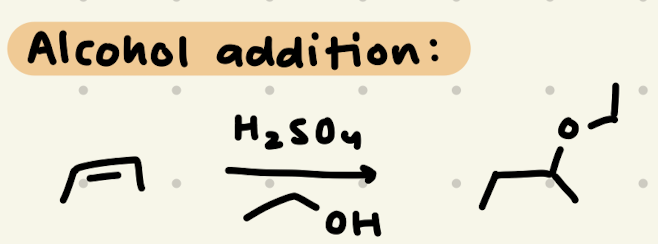
1) Adds to resonance stabilized carbocation
2) adds to more stable carbocation
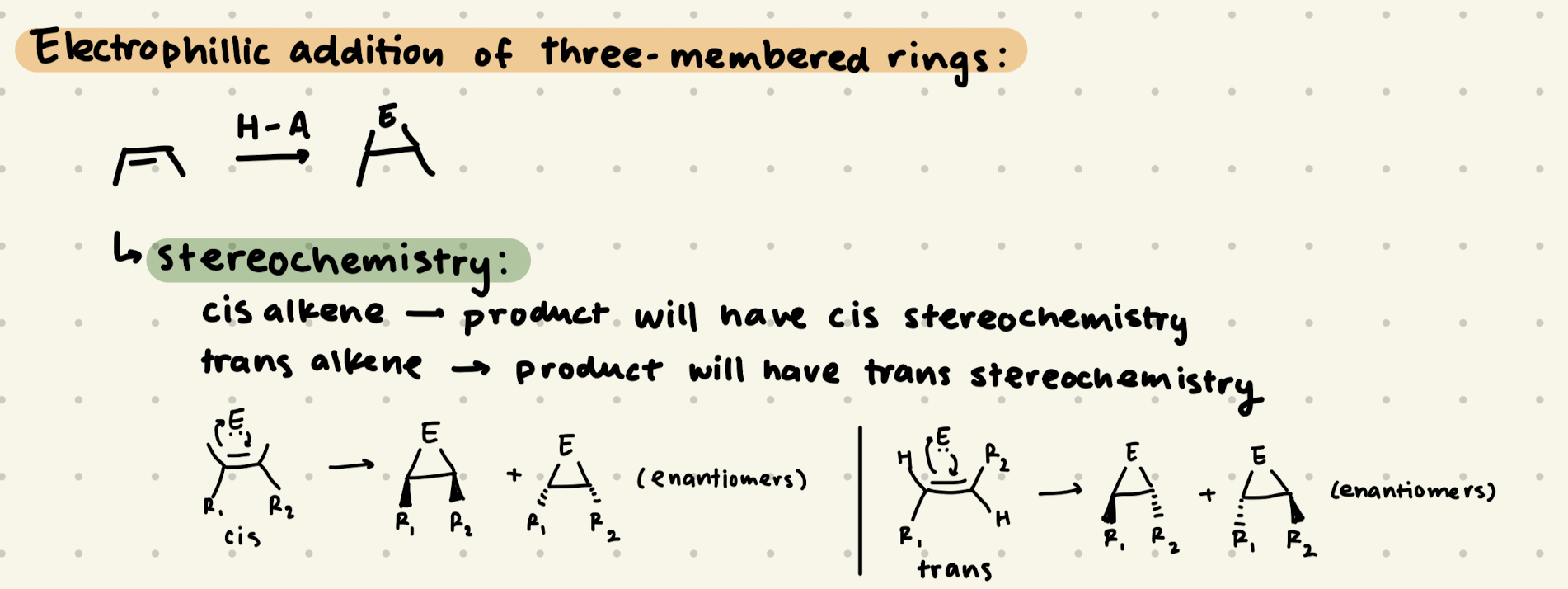
H-A
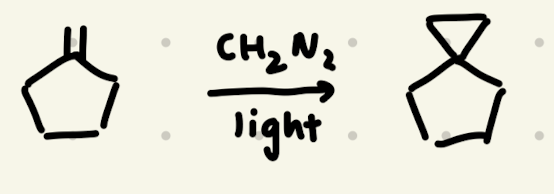
CH2N2, Light
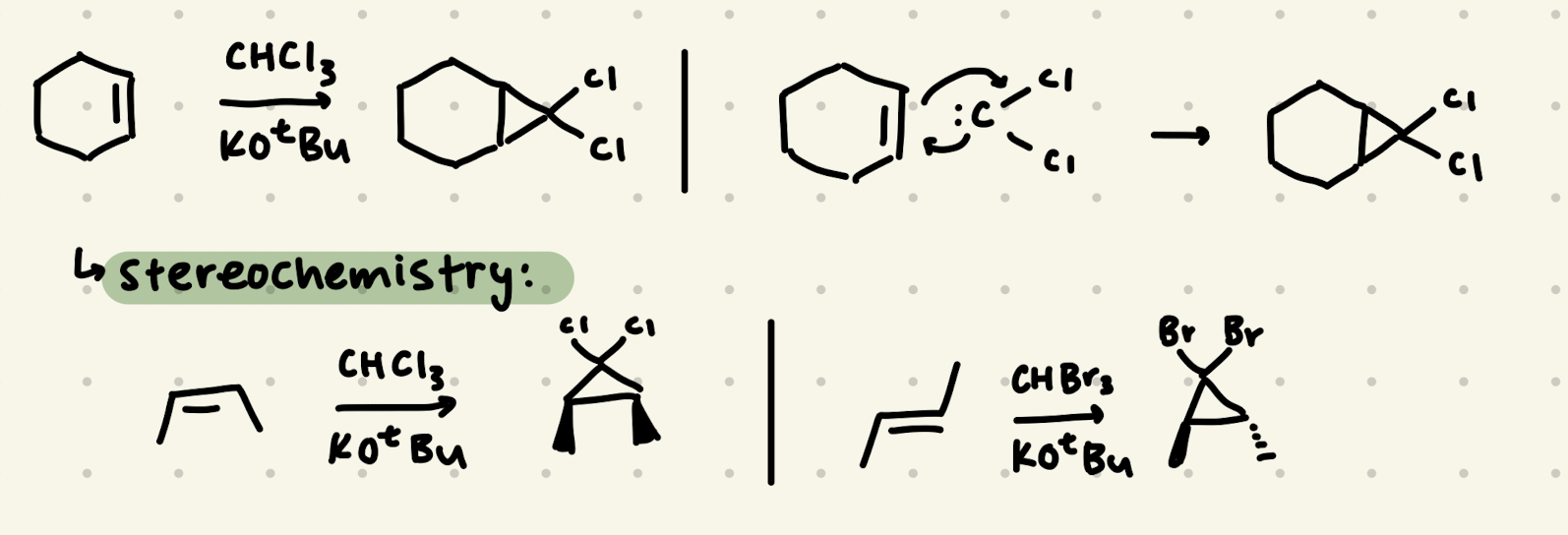
CHCl3, KOtBu
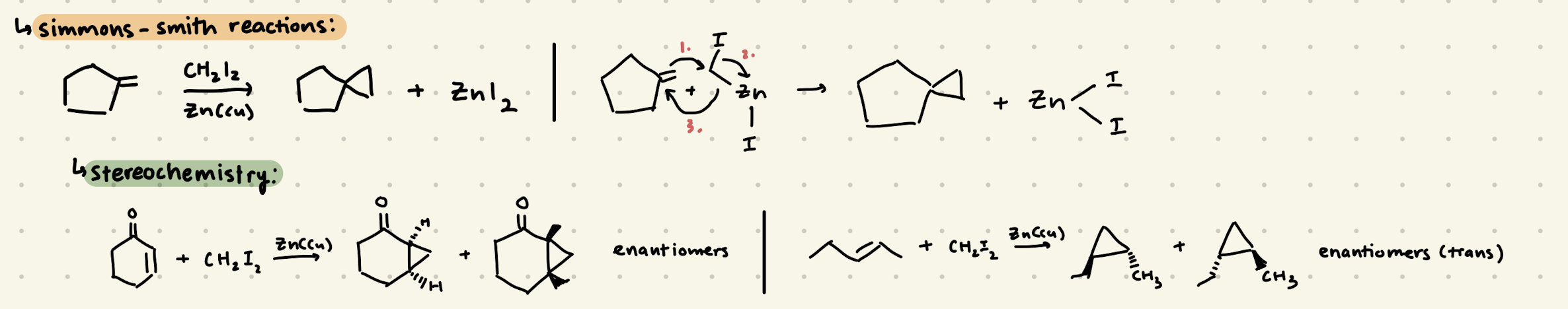
CH2I2, Zn(Cu)
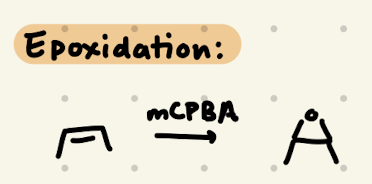
mCPBA
Epoxide Opening:
NaOR
H3O+
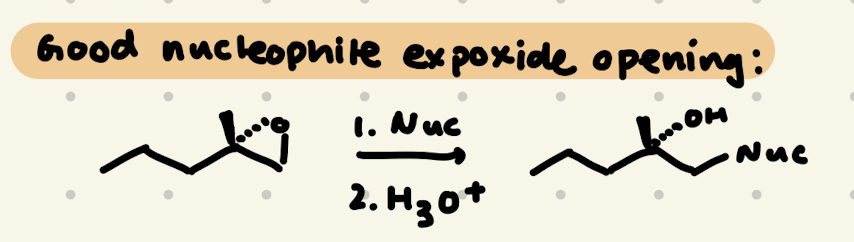
1) Nuc adds to less substituted carbon
2) Inversion of Stereochemistry at a chiral center
Epoxide Opening:
1) LiAlH4
2) H2O
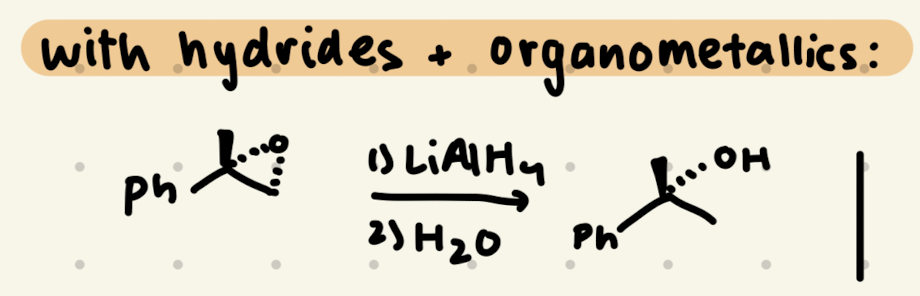
Epoxide Opening:
1) R-MgBr
2) H3O+
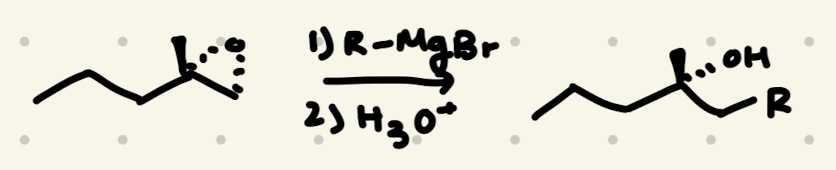
Epoxide Opening:
1) R-Li
2) H3O+
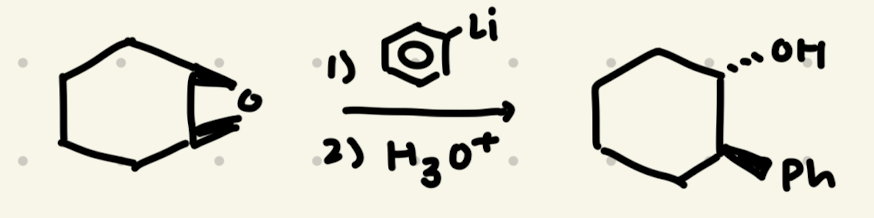
→ backside attack, makes enantiomers
Epoxide Opening:
H2SO4, CH3OH
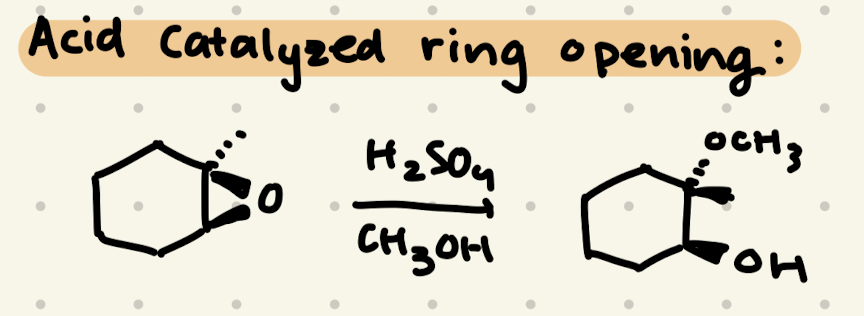
1) Add the nucleophile to the more substituted side
2) invert stereochemistry
Br2
Cl2, H2O
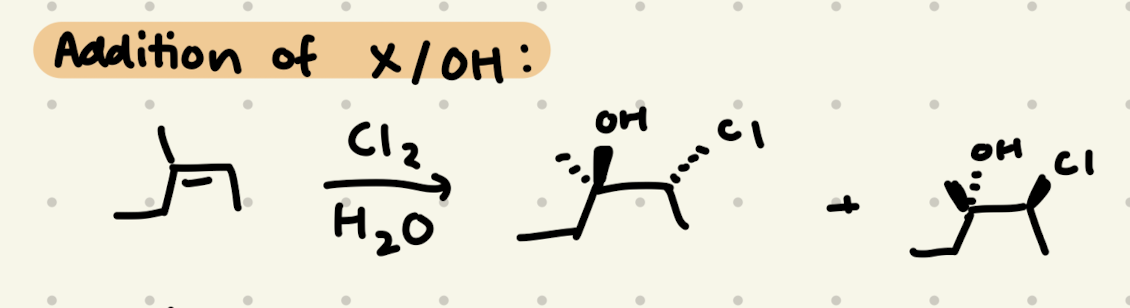
→ OH is added to the more substituted side
1) Hg(OAc)2, H2O
2) NaBH4
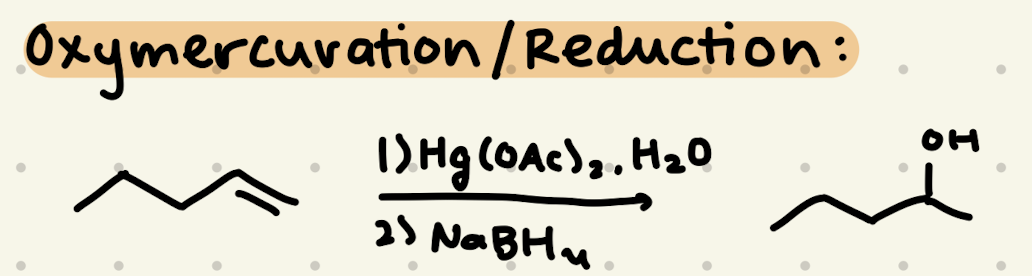
→ OH is added to the more stable side
1) BH3
2) H2O2, NaOH
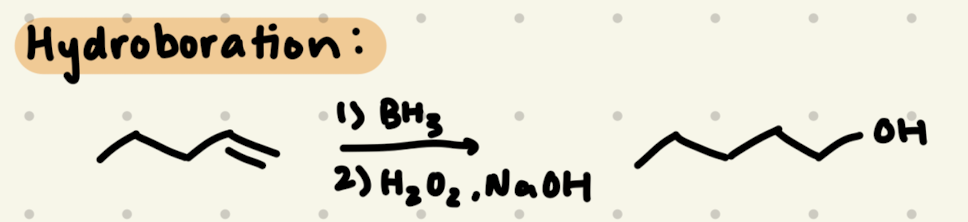
1) OH is added to the less substituted side
2) Syn addition of H & OH
1) OsO4
2) NaHSO3, H2O
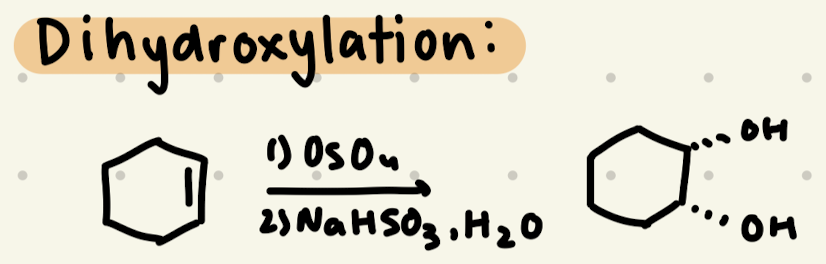
→ Syn addition of OH
NaIO4
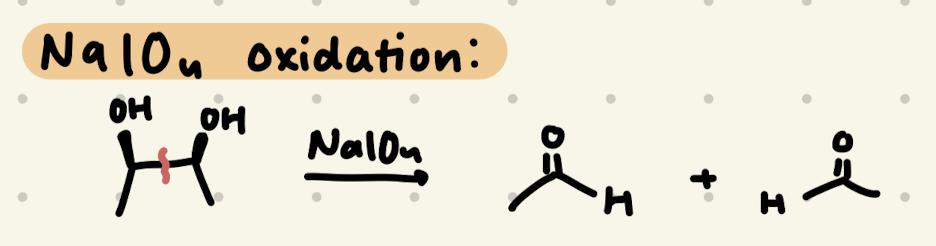
→ Carbon between OH becomes H
→ OH needs to be syn
1) O3
2) Zn
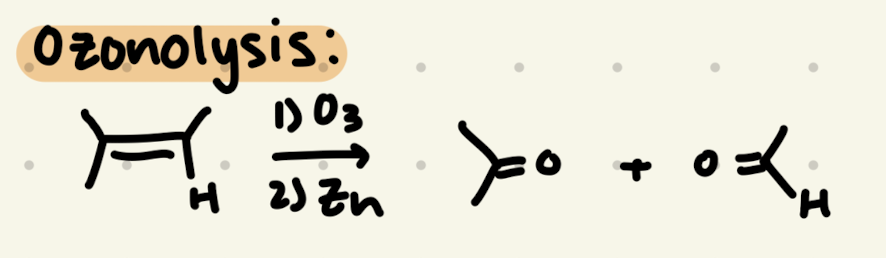
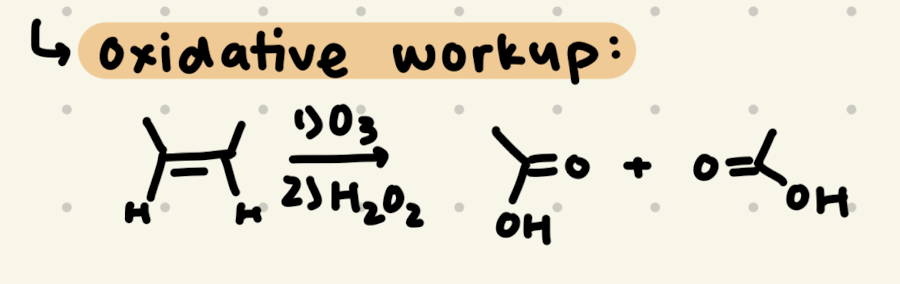
1) O3
2) H2O2
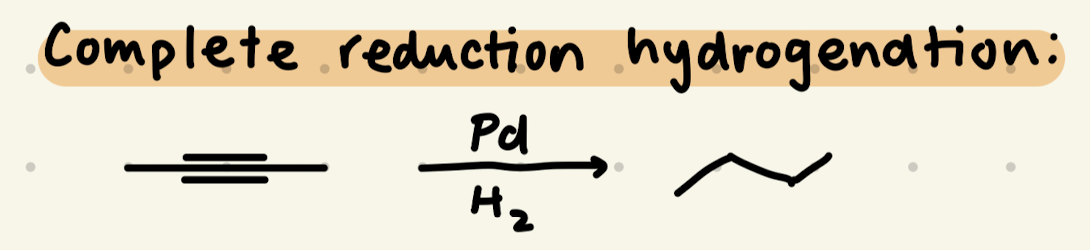
Pd, H2
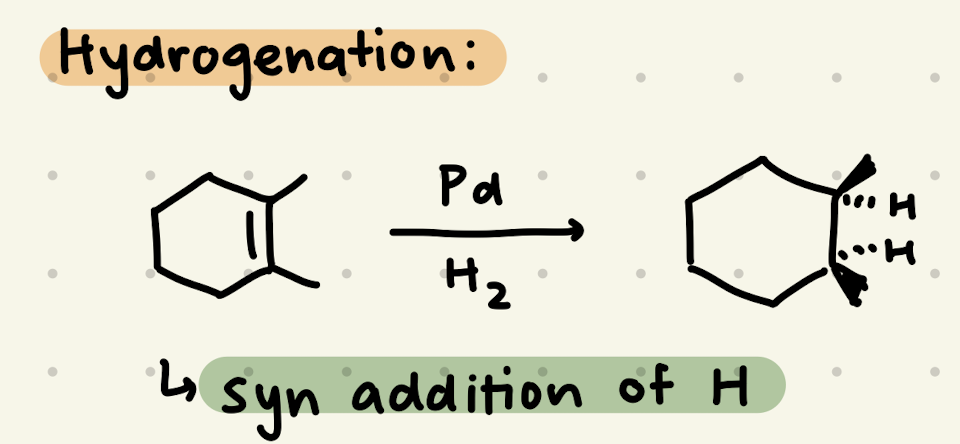
Pd, H2, Quinoline

→ makes cis alkenes
Na or Li, NH3(l)
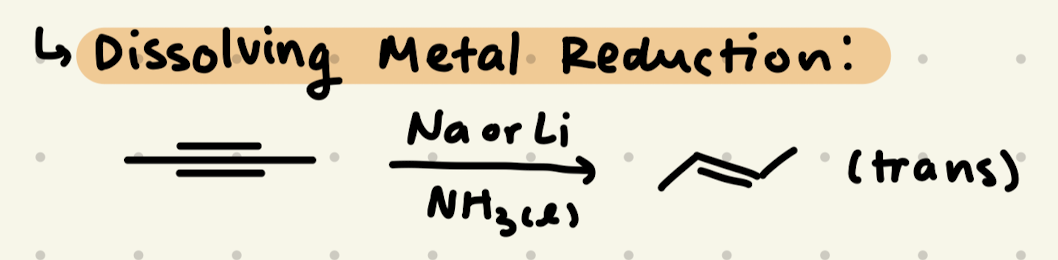
→ makes trans alkenes
1) LiAlH4
2) H3O+

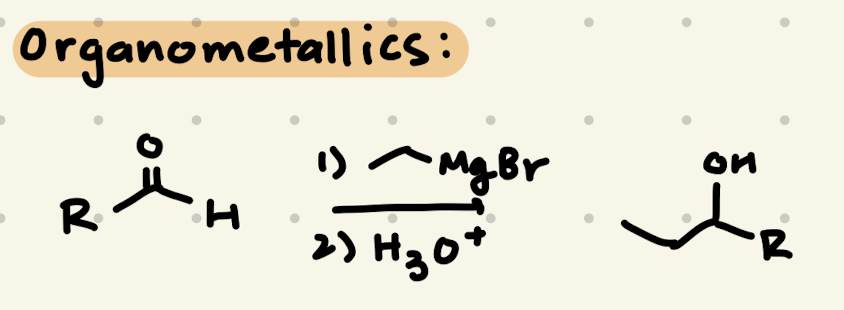
In an Aldehyde/Ketone:
1) R-MgBr
2) H3O+
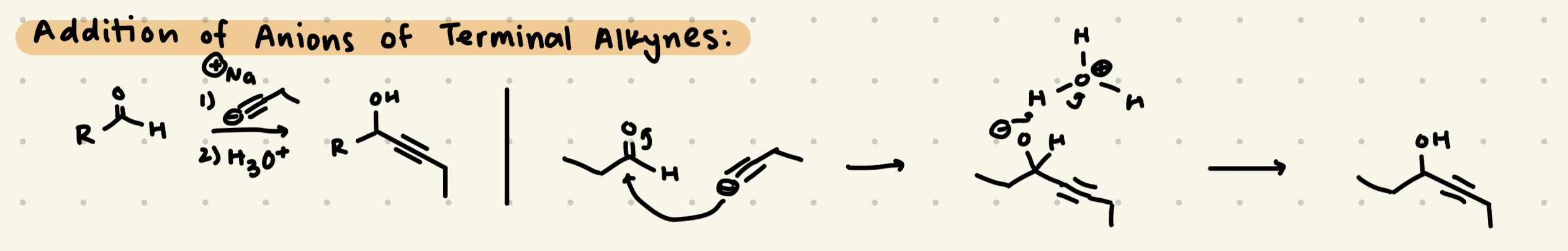
1) Na-Terminal Alkyne
2) H3O+
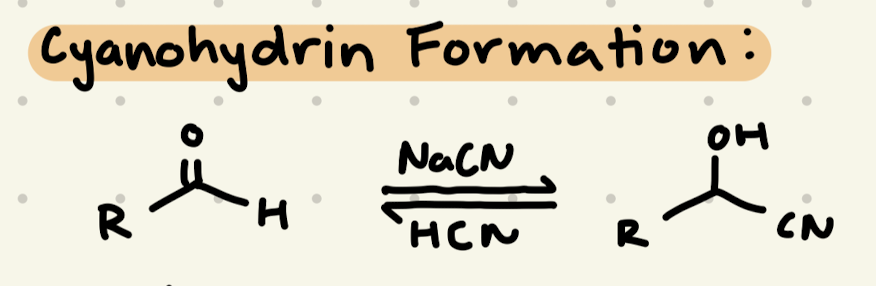
NaCN, HCN
→ works for both aldehydes and ketones
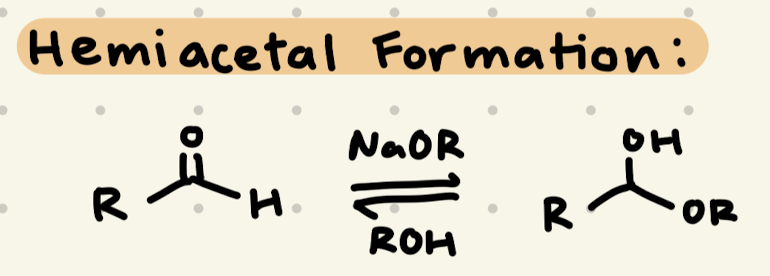
NaOR, ROH
PH3P+- -CH2
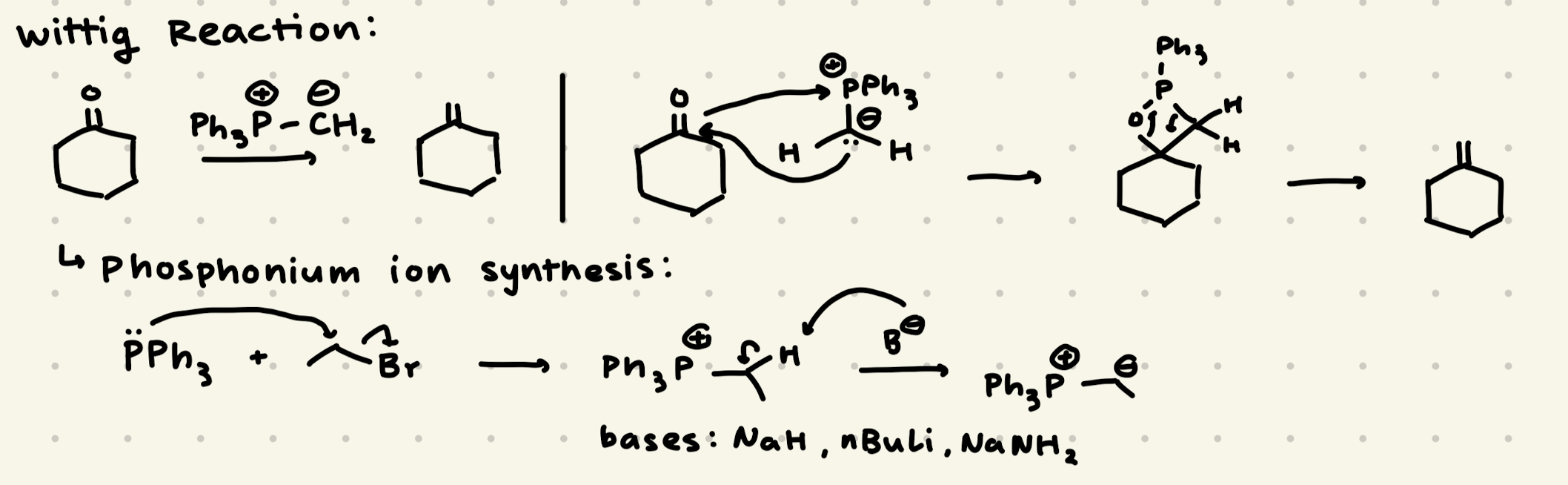
→ Unstabilized Ylide = Z-Alkene
→ Stabilized Ylide = E-Alkene
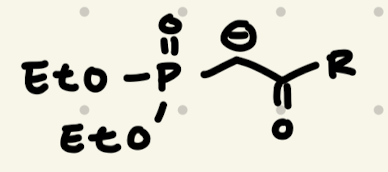
1) NaOCH3
2) aldehyde/ketone
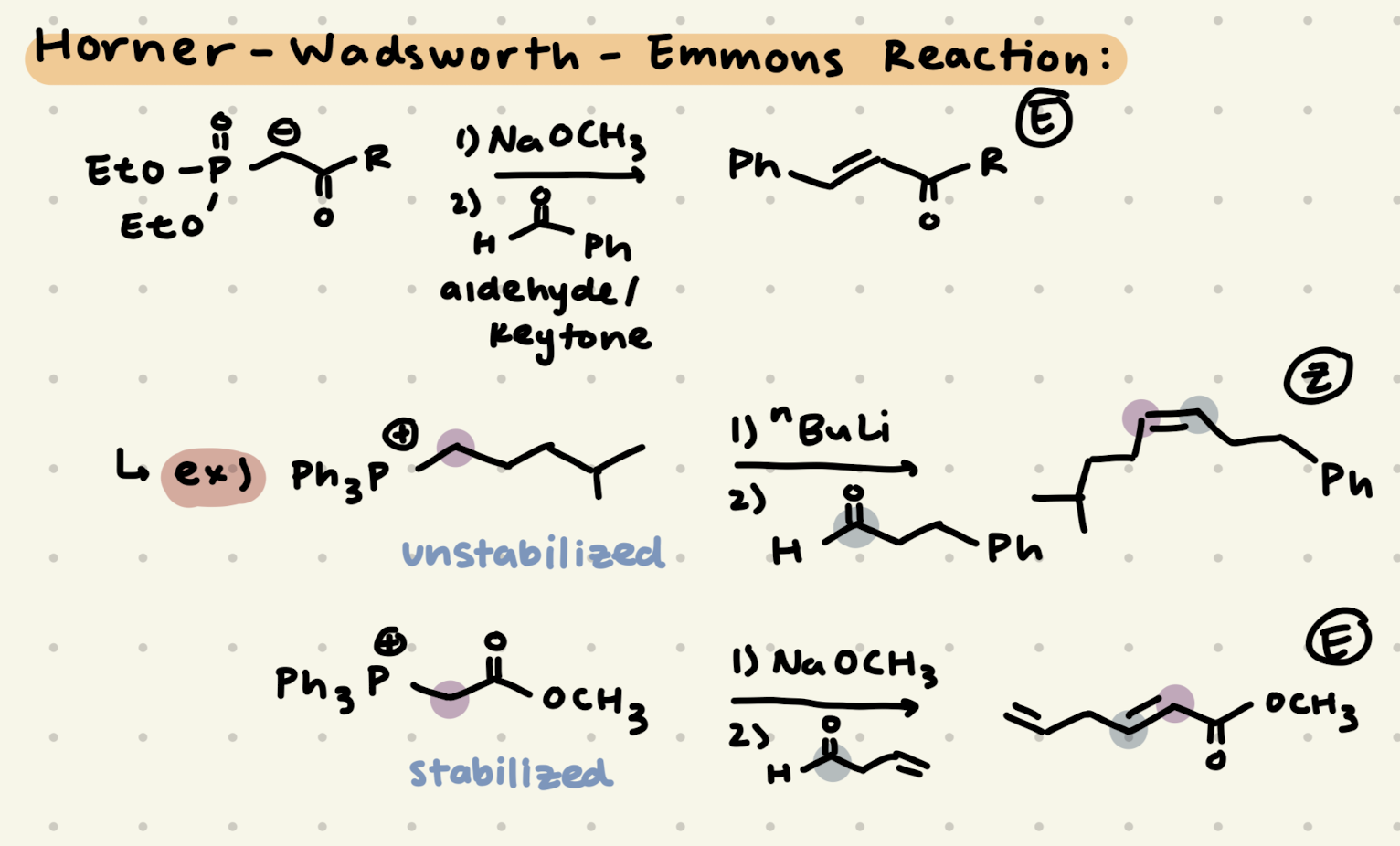
HCl, H2O
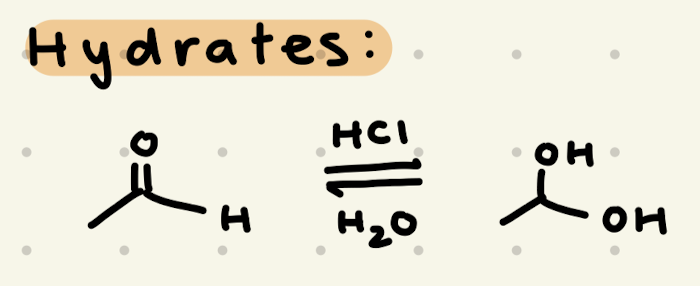
HCl, EtOH
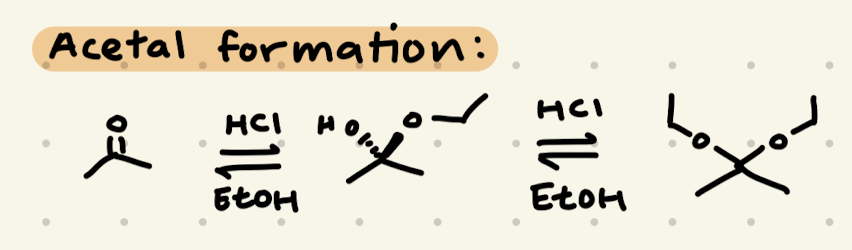
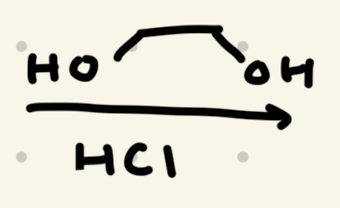
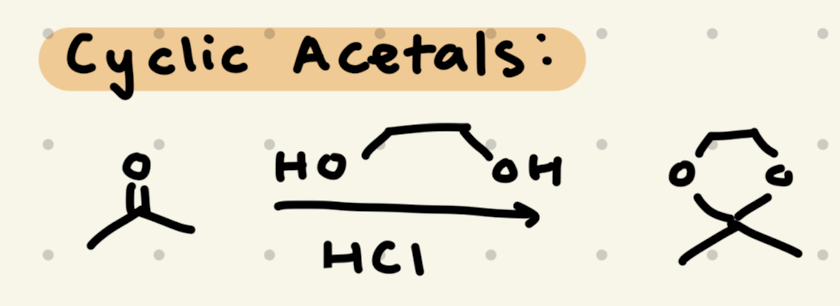
→ works as a protecting group for aldehydes/ketones
cat. HCl, R-NH2
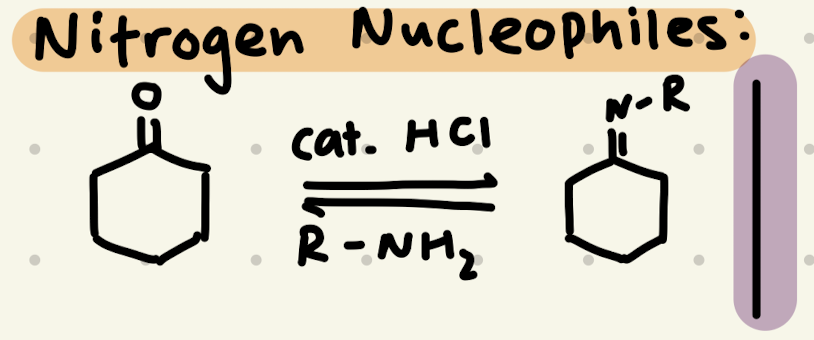
→ can also be cat. acetic acid
→ can be used on both aldehydes and ketones
cat. HCl, Secondary Amine
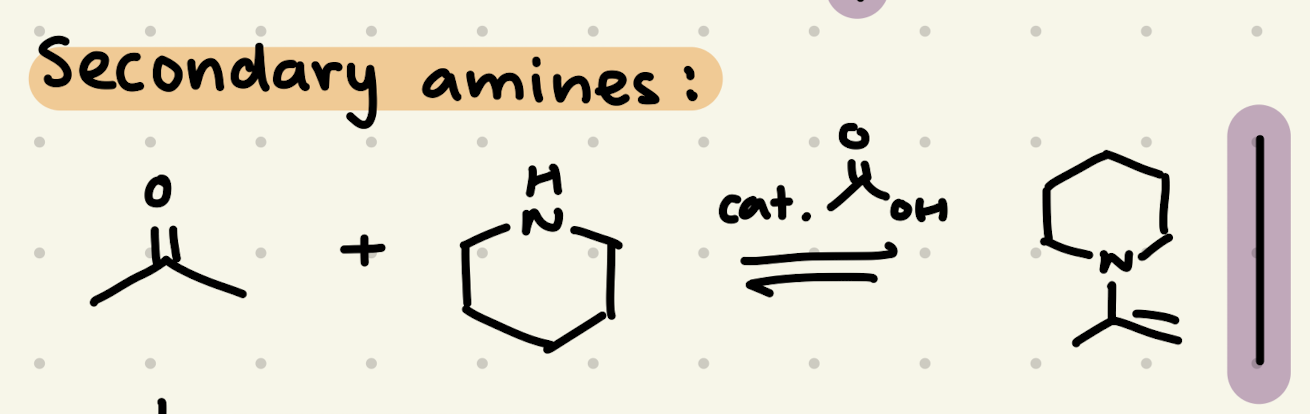
H2N-R
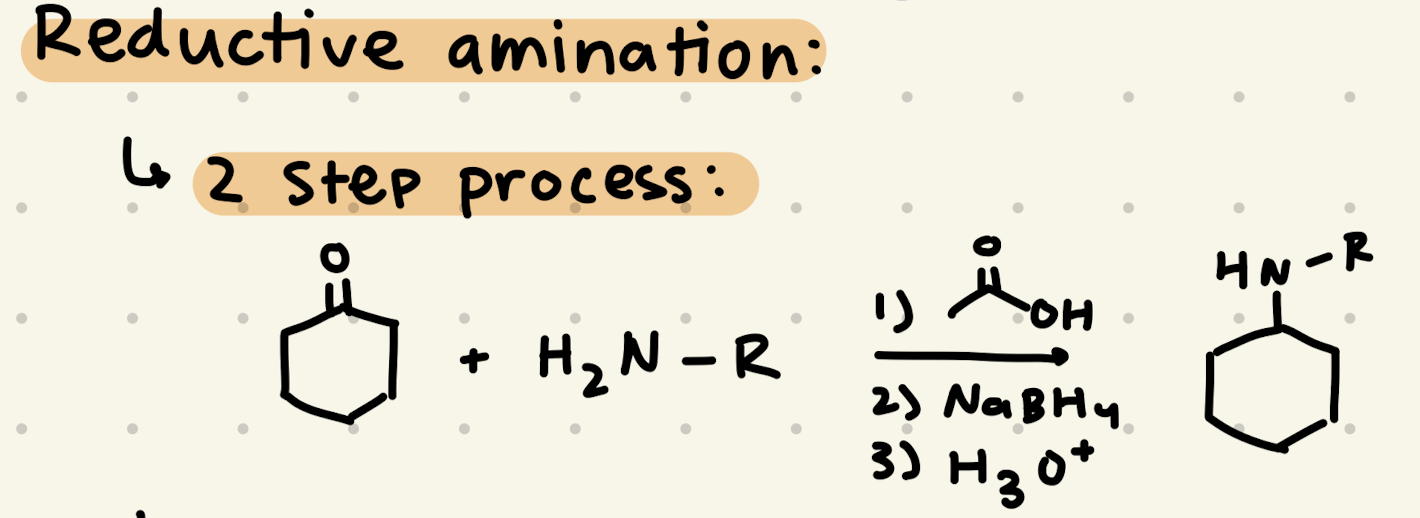
1) acetic acid
2) NaBH4
3) H3O+
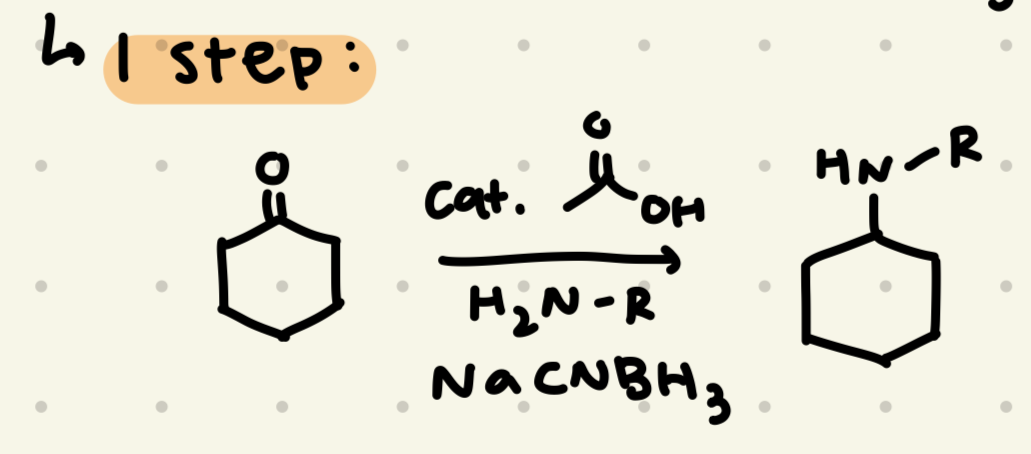
cat. acetic acid
H2N-R
NaCNBH3
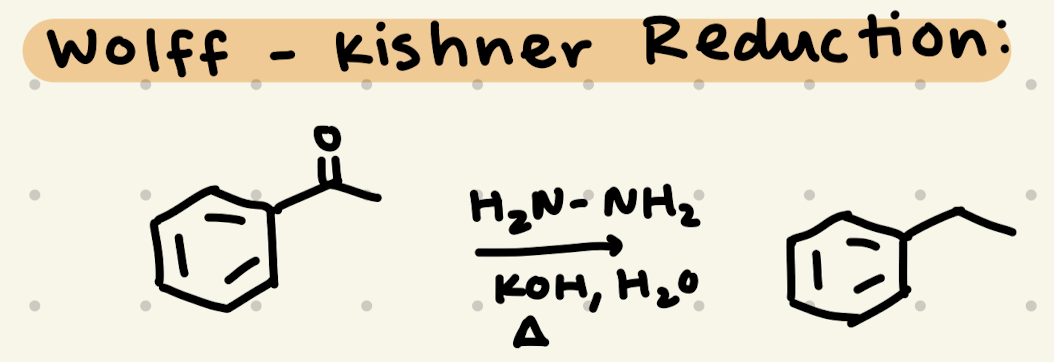
H2N-NH2
KOH, H2O
Heat
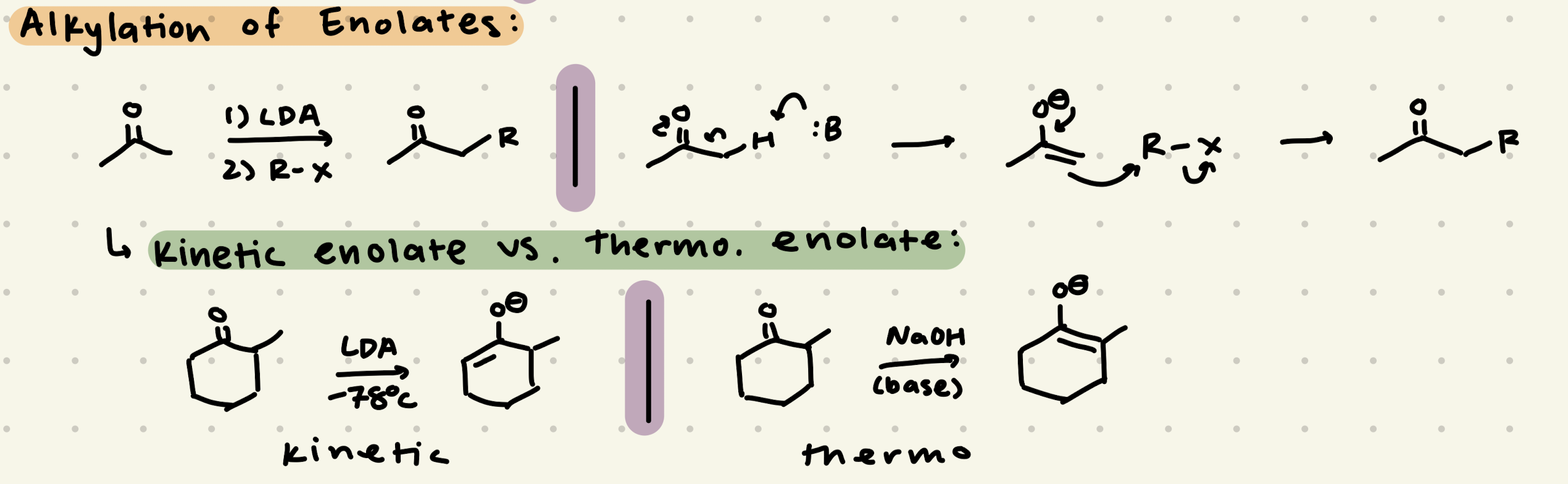
1) LDA or NaOH
2) R-Br
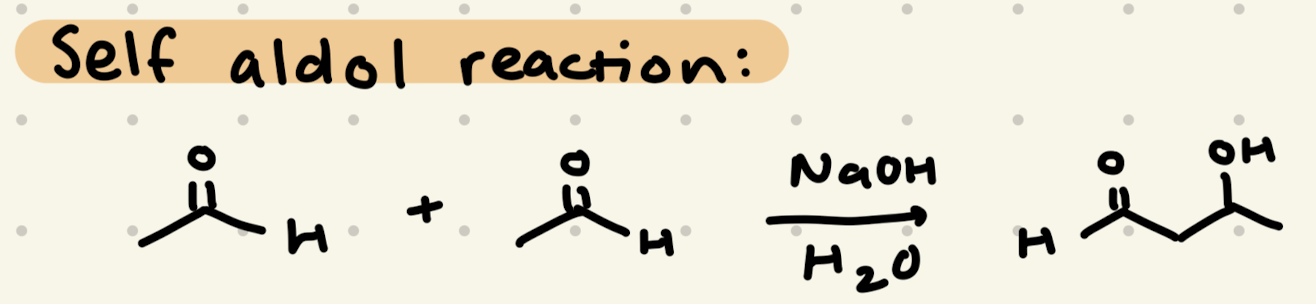
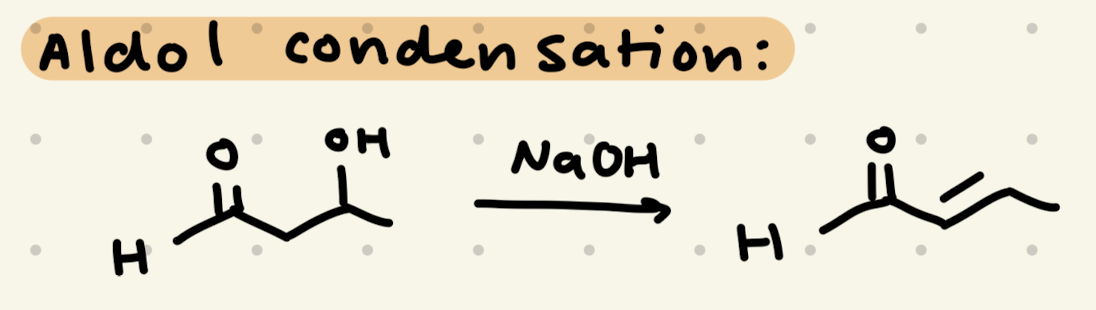
NaOH, H2O
or
HCl
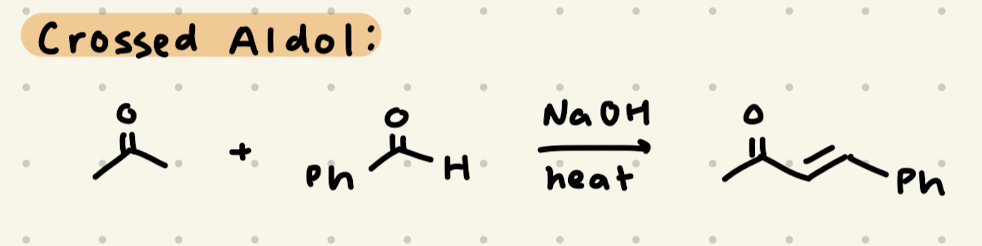
NaOH, heat
NaOMe, CH3OH
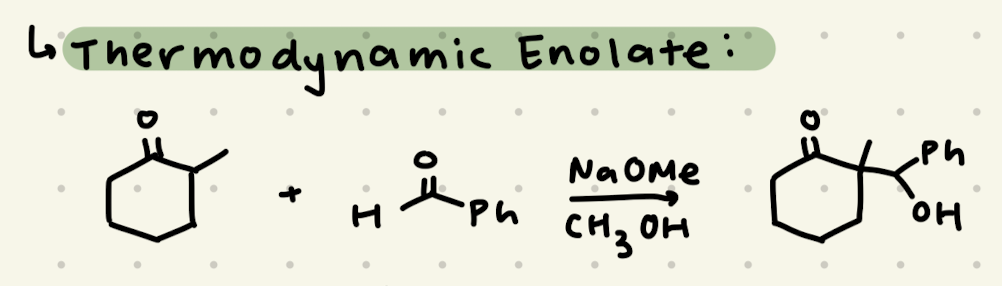
→ More substituted side
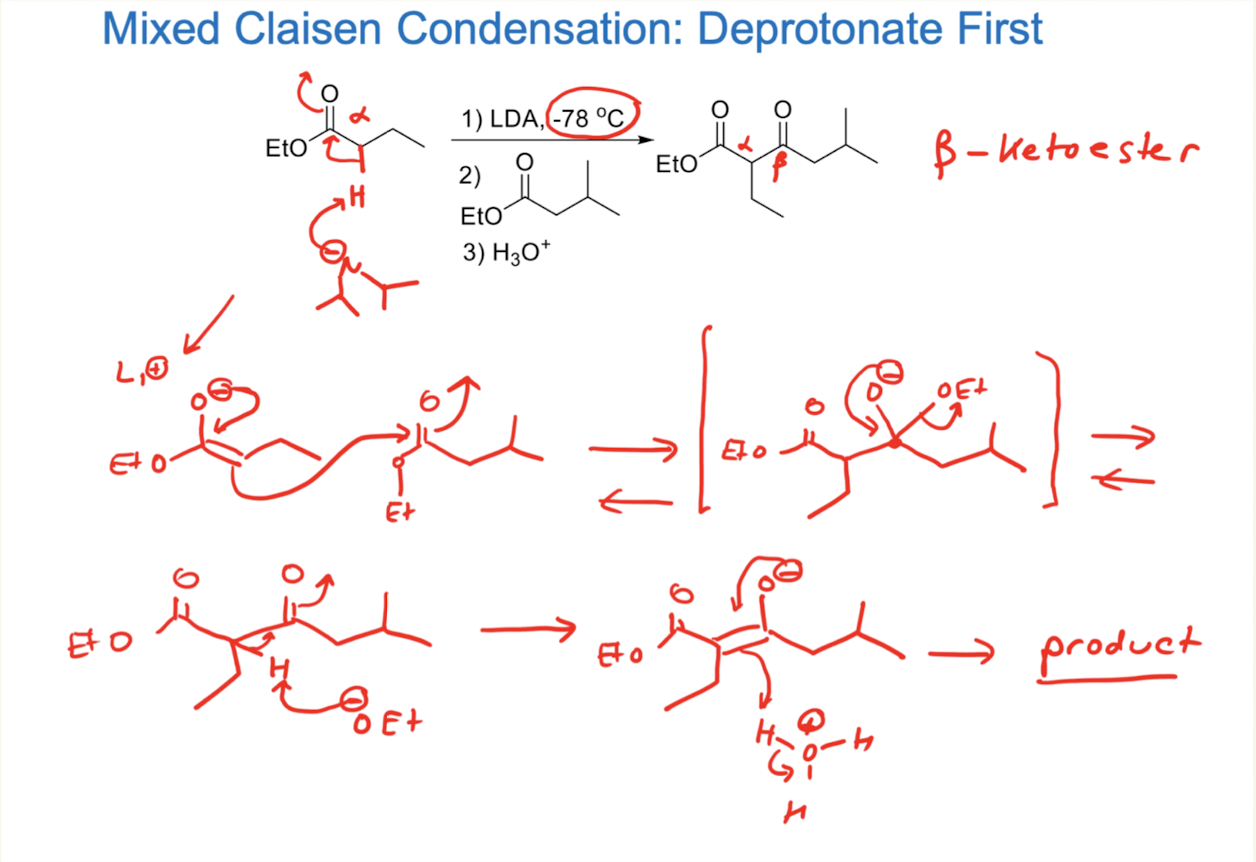
1) LDA, -78ºC
2) H3O+
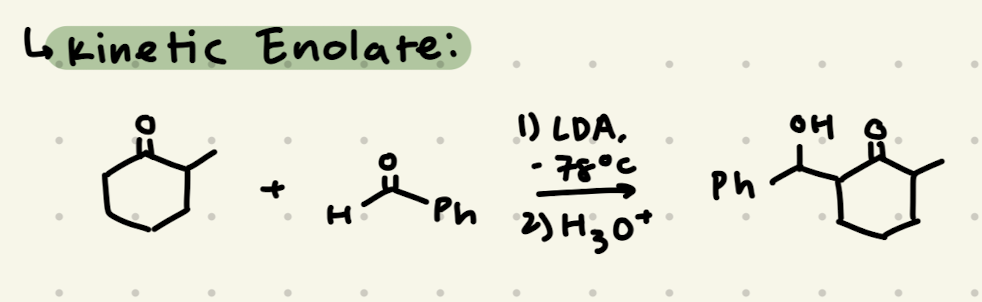
→ Less substituted sidee
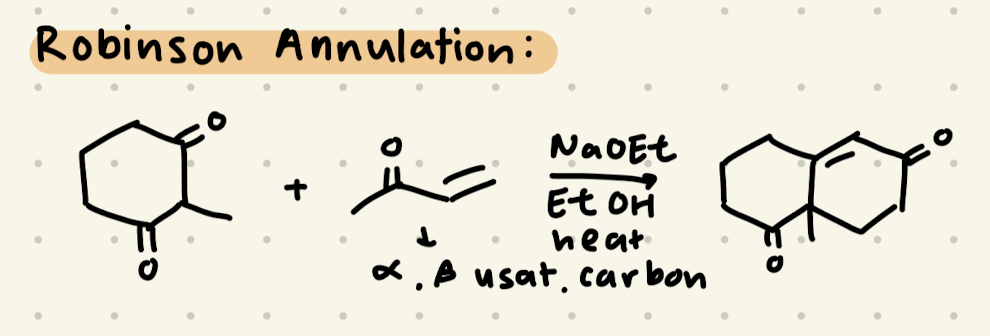
Explain 1,2 vs. 1,4 addition
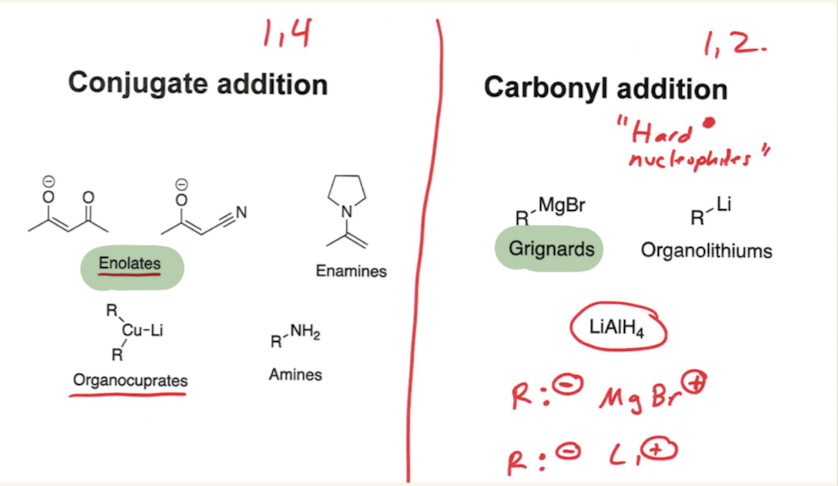
→ this occurs in alpha, beta unsaturated carbons
NaOH, EtOH
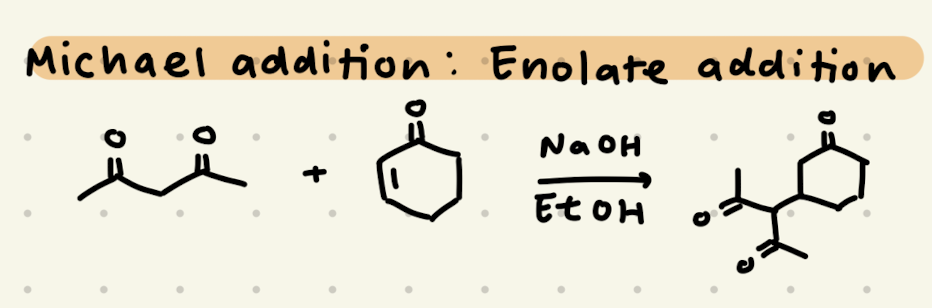
NaOH, EtOH, heat
Reduction: Catalytic Hydrogenation (H2, Pd/C/Pt)
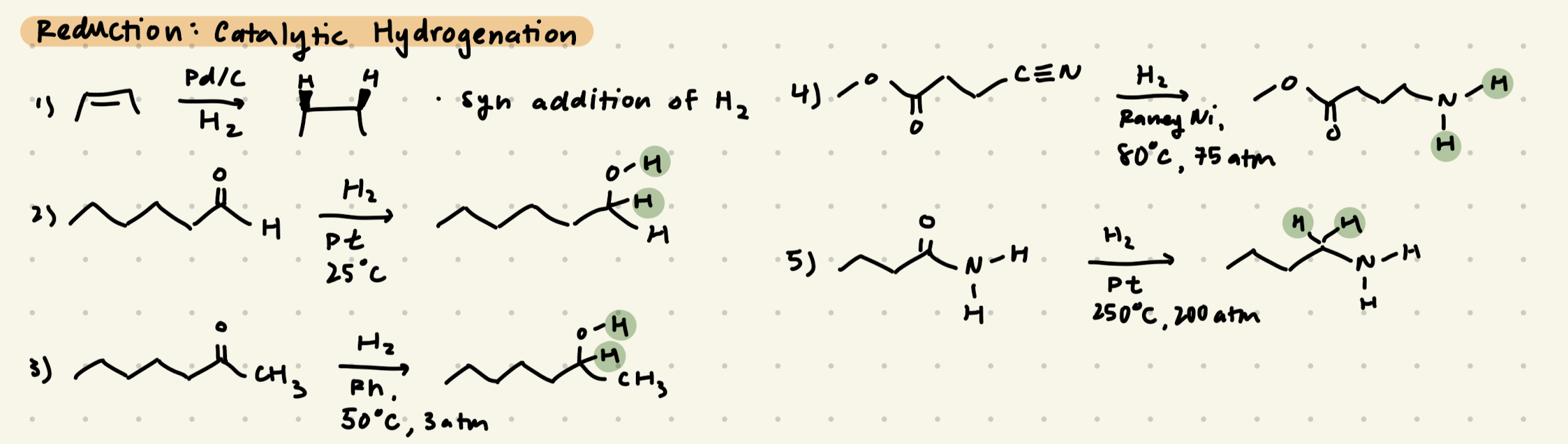
→ only reduces aldehydes, but not ketones
CrO3, H2SO4, H2O
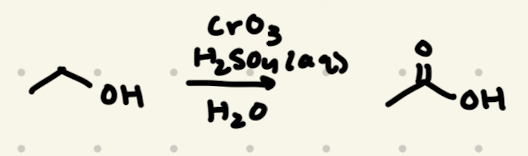
→ no reactions for 3º -OH
→ no new carbons added
1) (COCl2), DMSO
2) NEt3
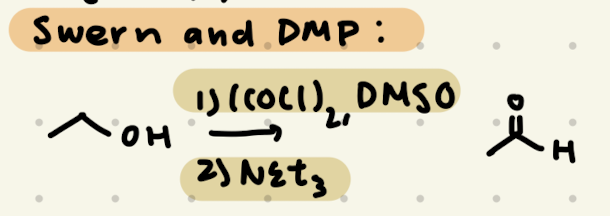
→ no reaction for 3º -OH
DMP
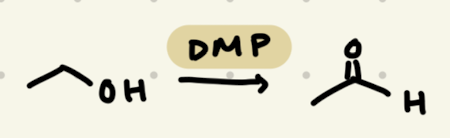
→ does not work for 3º -OH
TMSCl
TBAF
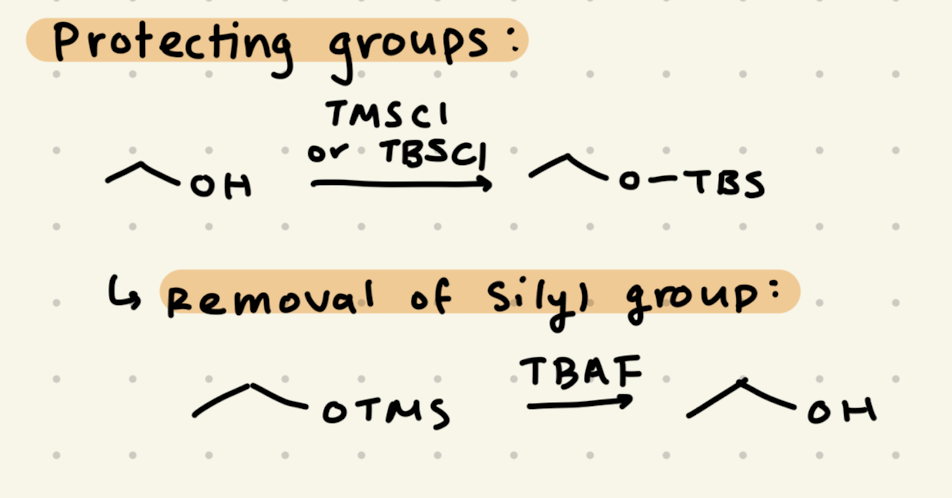
→ protecting group for alcohol
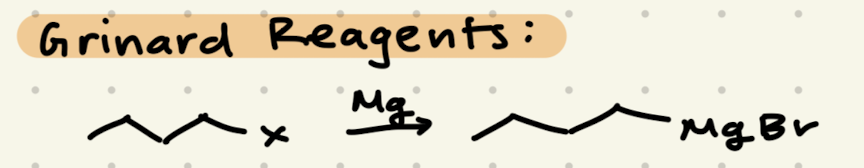
Mg
1) Mg
2) CO2
3) H3O+
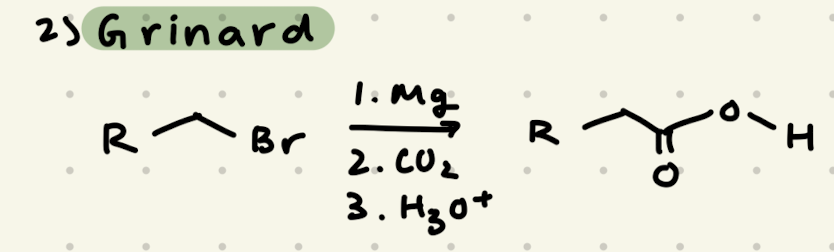
→ added new carbons
SOCl2
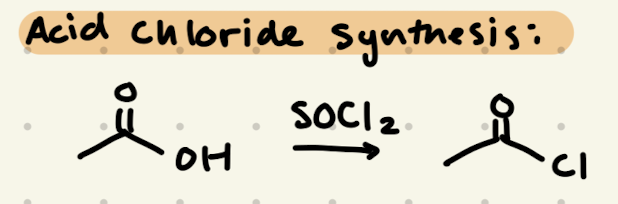
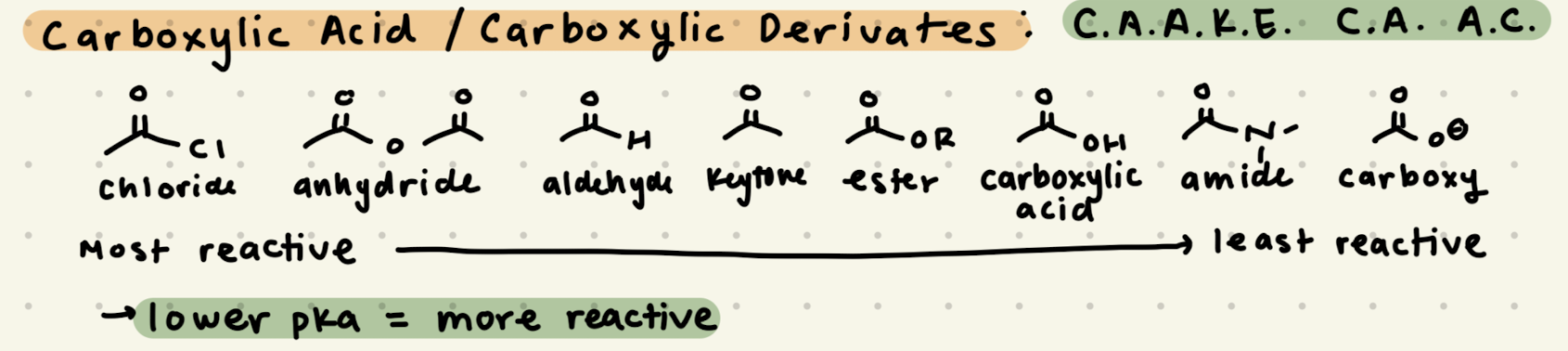
C.A.A.K.E. C.A. A.C.
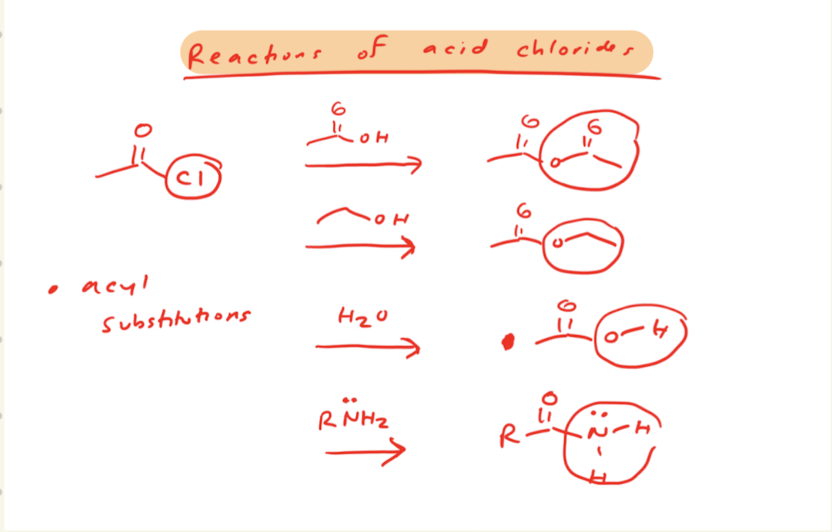
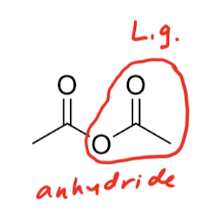
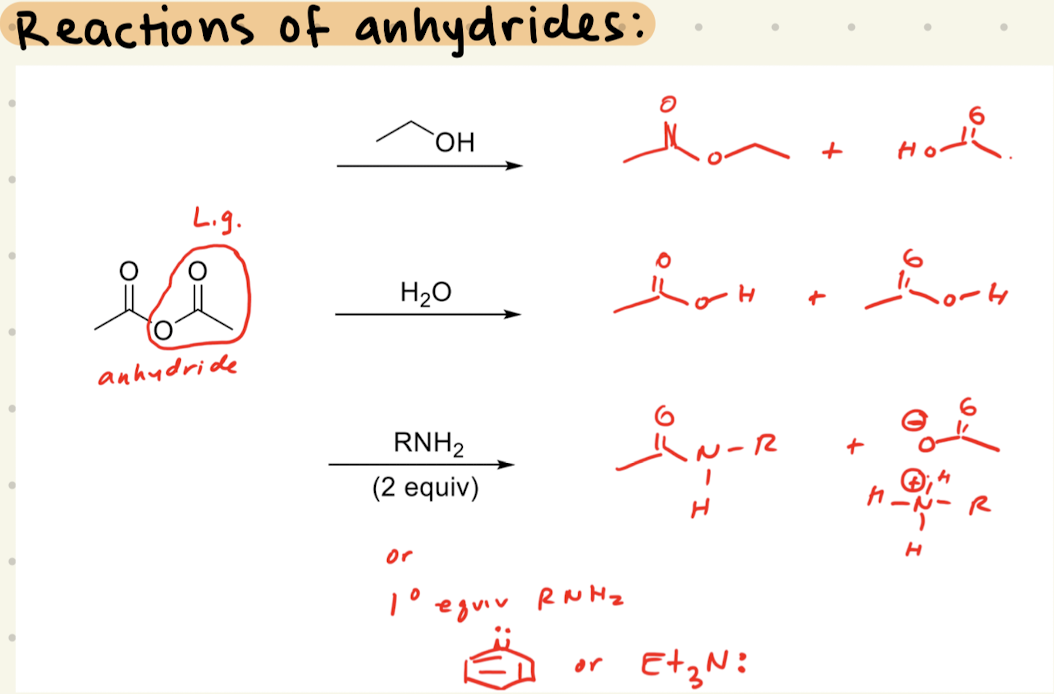
Ester
NaOH, H2O, HCl

Ester
H2SO4 or H3O+
R-OH
Ester
R-NH2, heat
DCC, R-NH2, NEt3
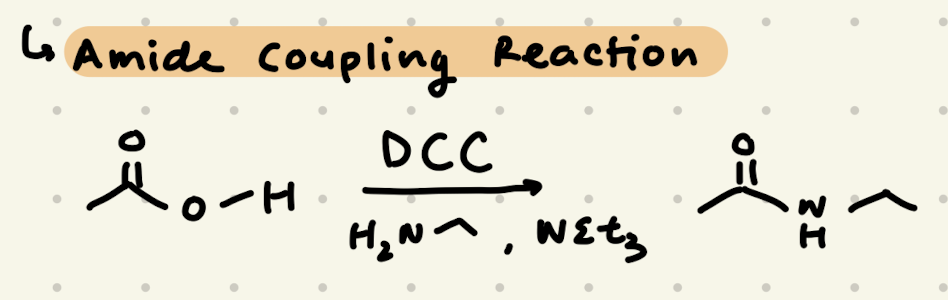
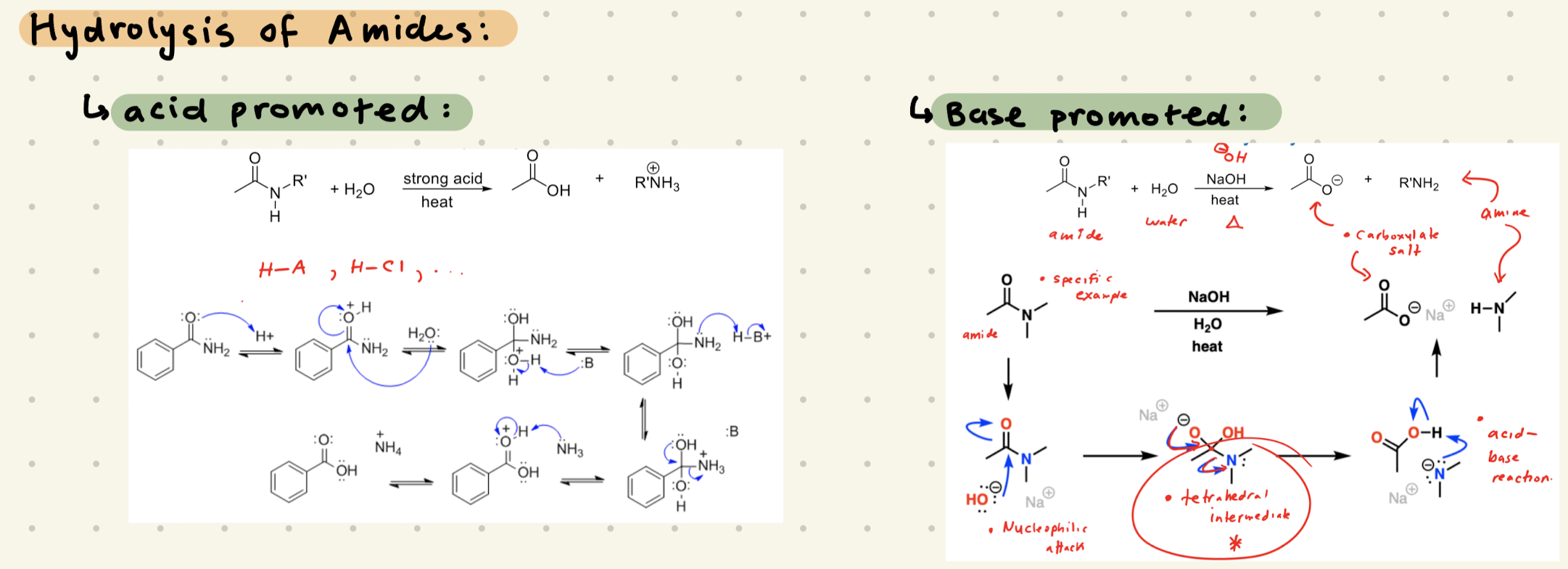
Amide, H2O
HCl or NaOH, heat
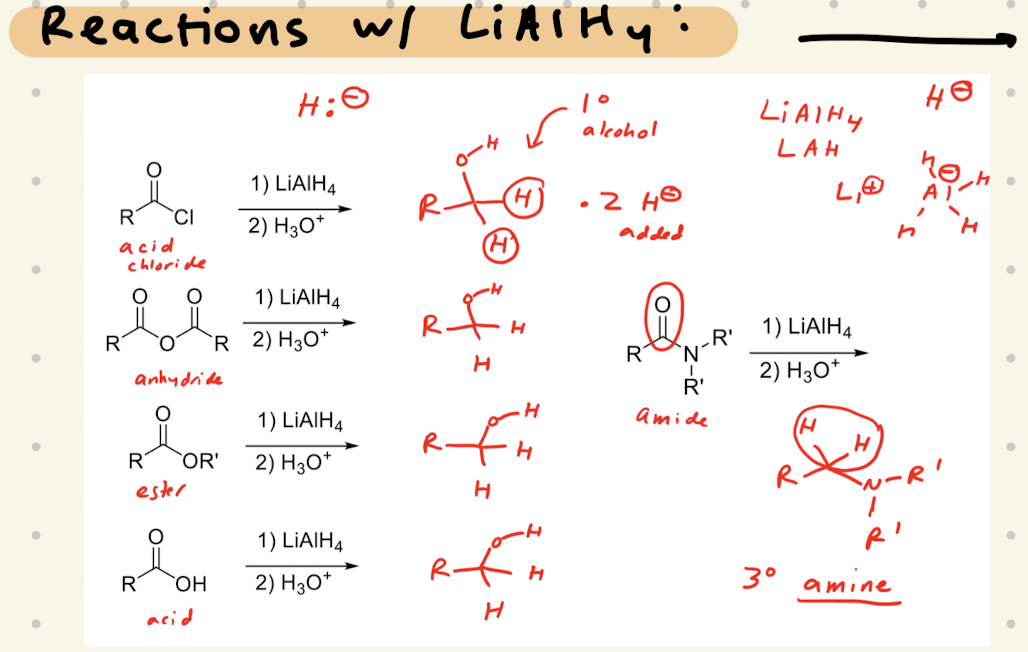
1) LiAlH4
2) H3O+

1) NaBH4
2) H3O+
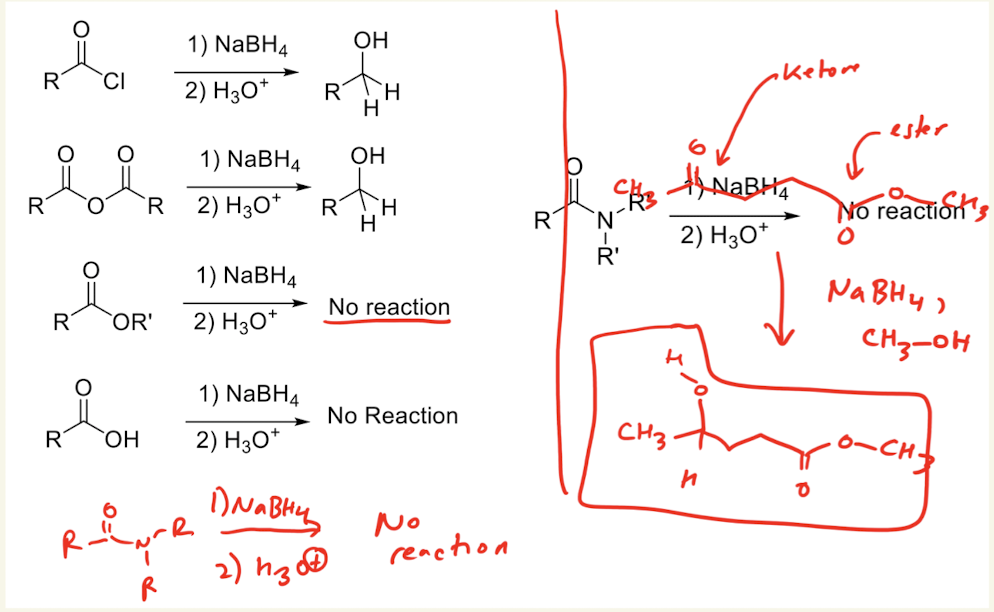
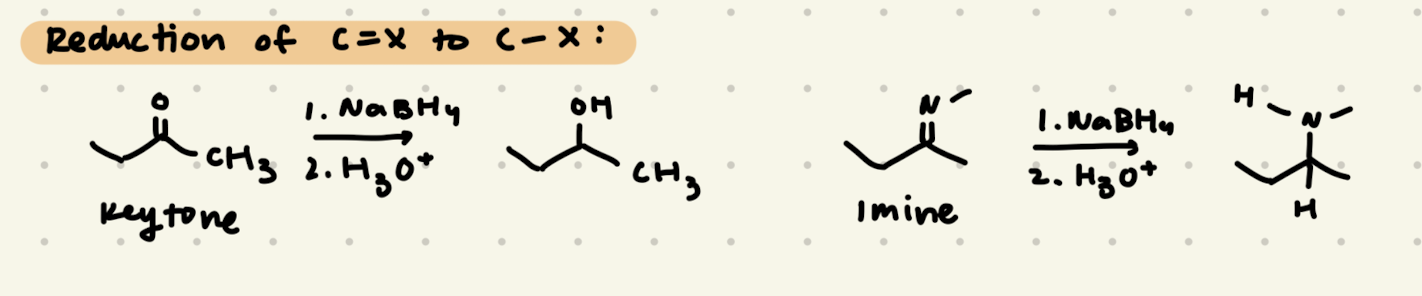
→ reacts with ketones and aldehydes, but not esters
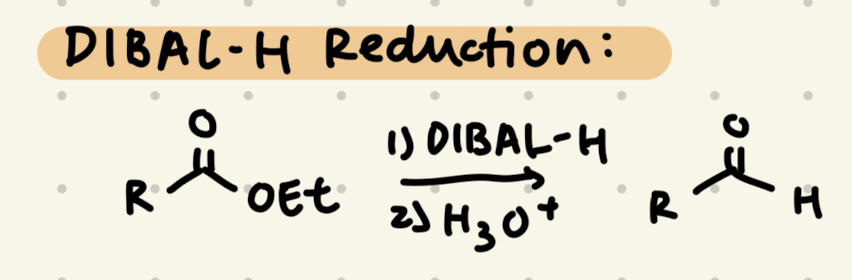
1) DIBAL-H
2) H3O+
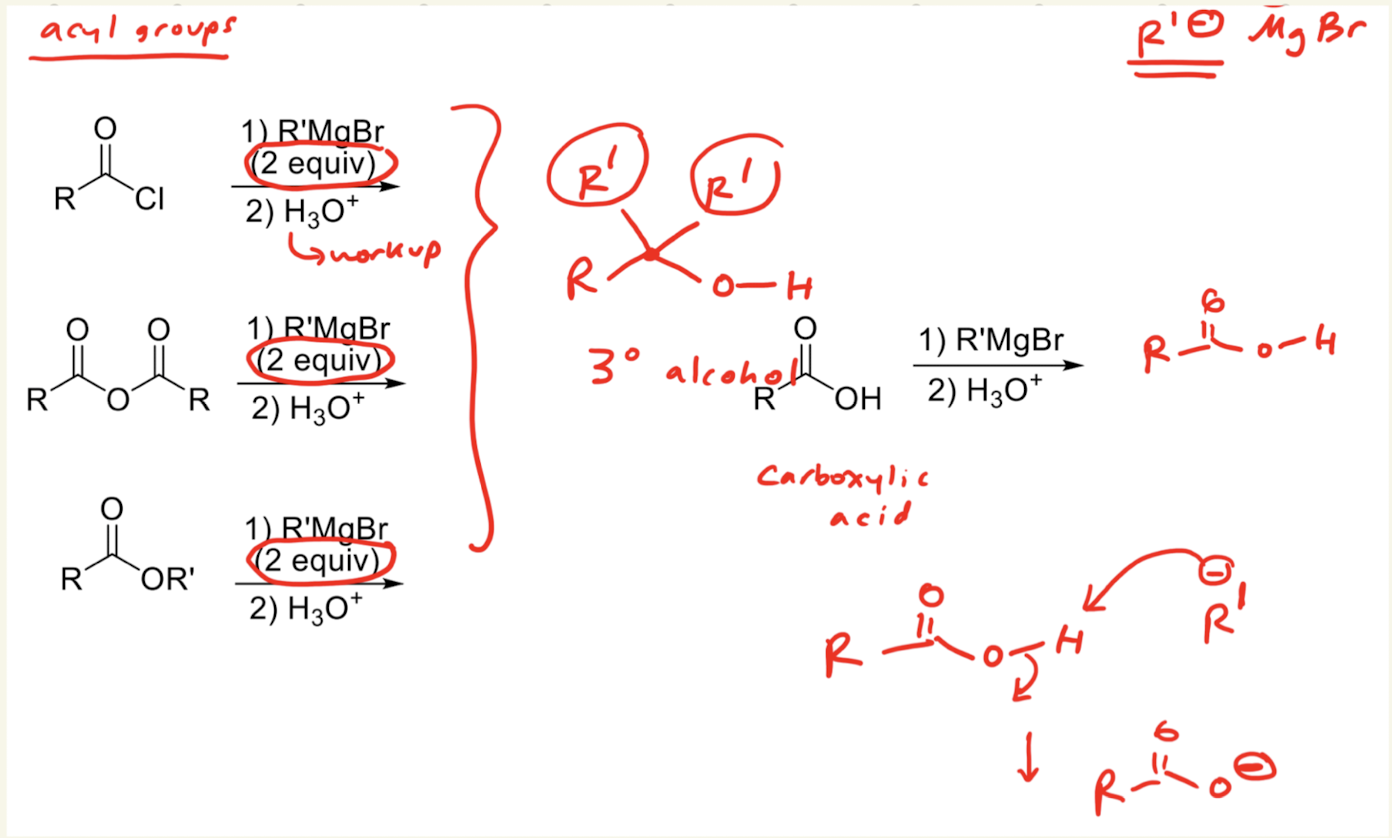
1) R’MgBr (excess)
2) H3O+
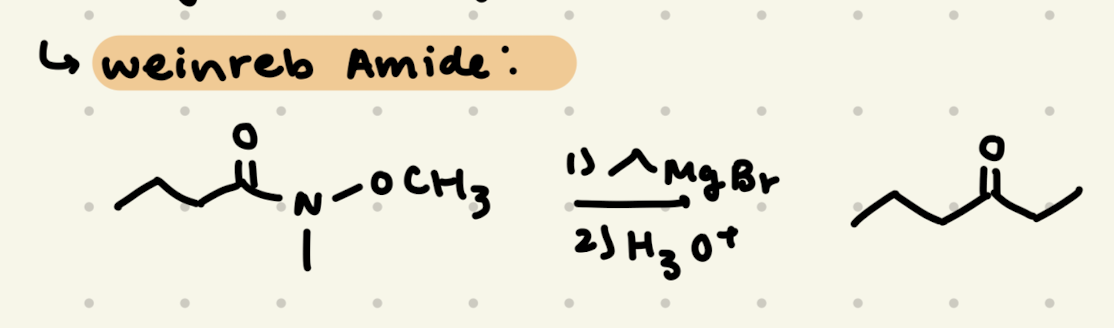
Amide:
1) CH3-MgBr
2) H3O+
1) NaOEt, EtOH
2) H3O+
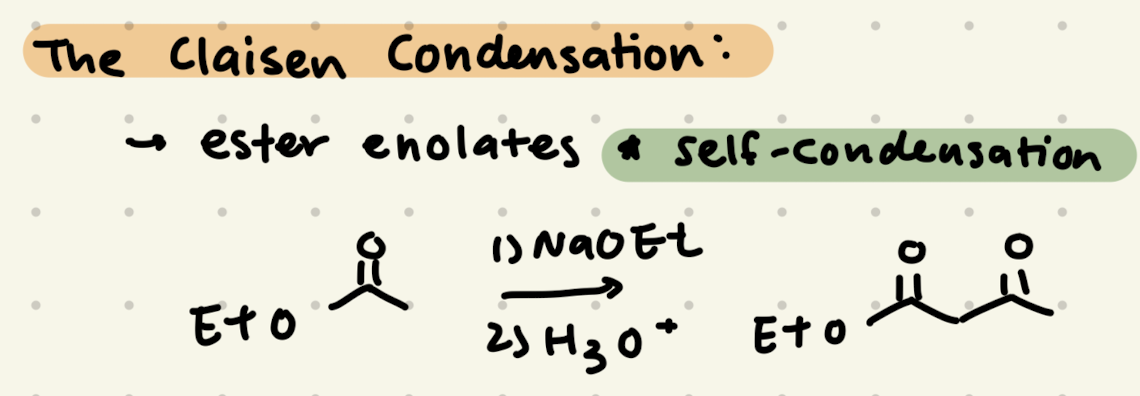
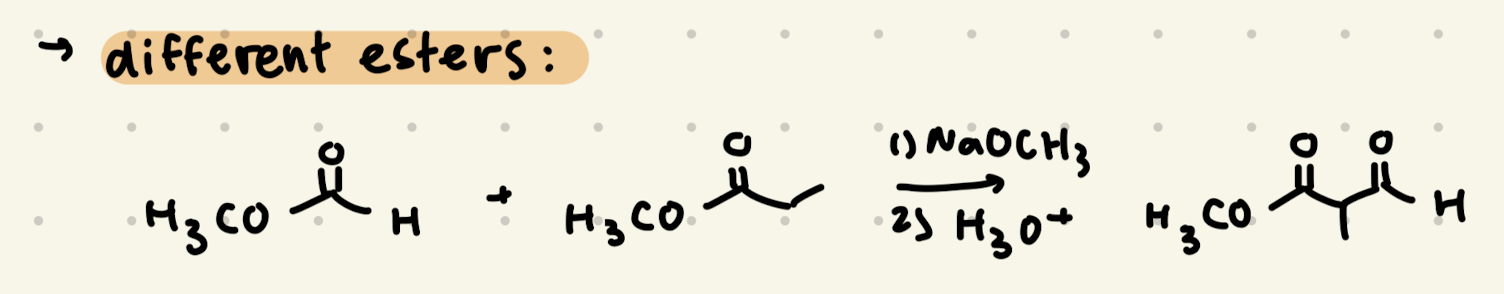
1) NaOCH3, CH3OH
2) H3O+
Claisen Condensation
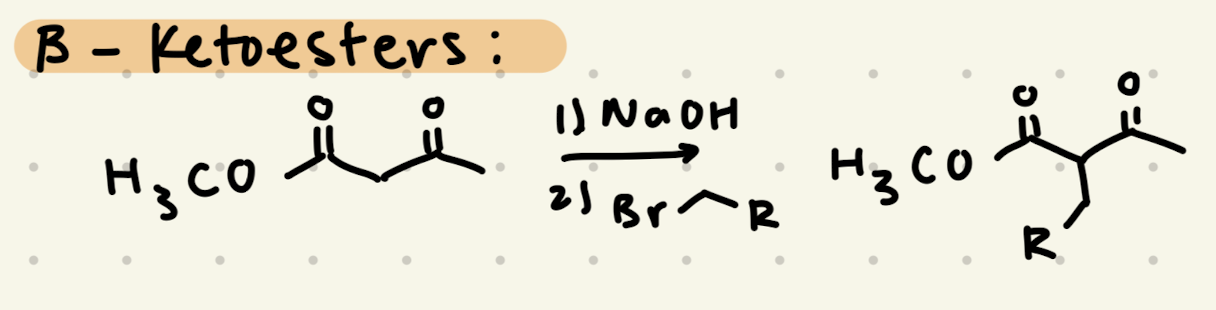
1) NaOH
2) Br-R
1) H2SO4, H2O
2) heat
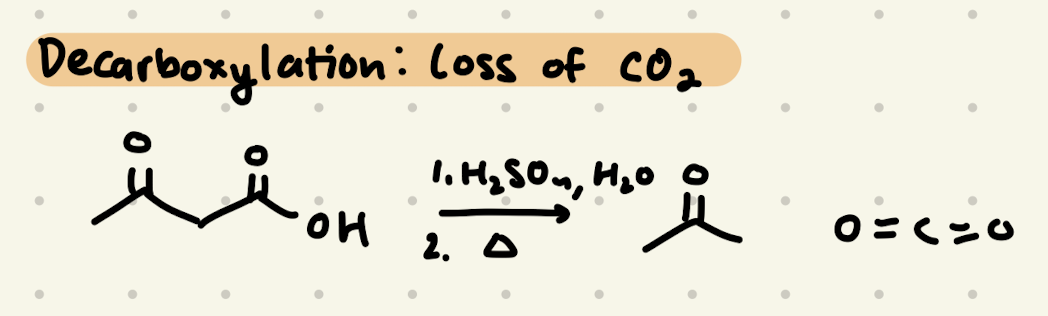
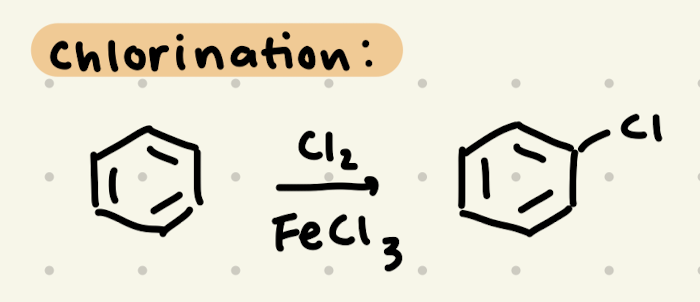
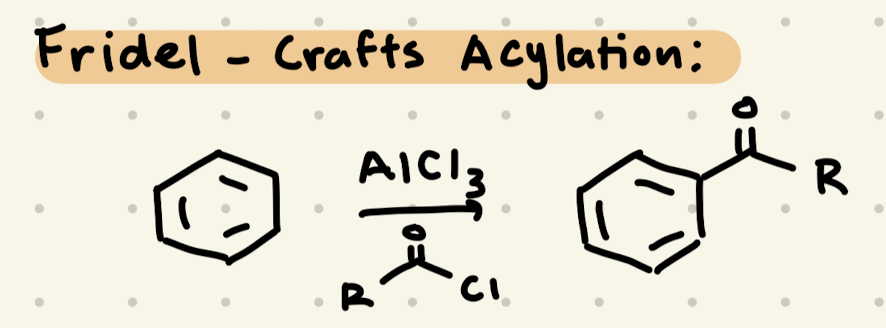
Cl2, FeCl3
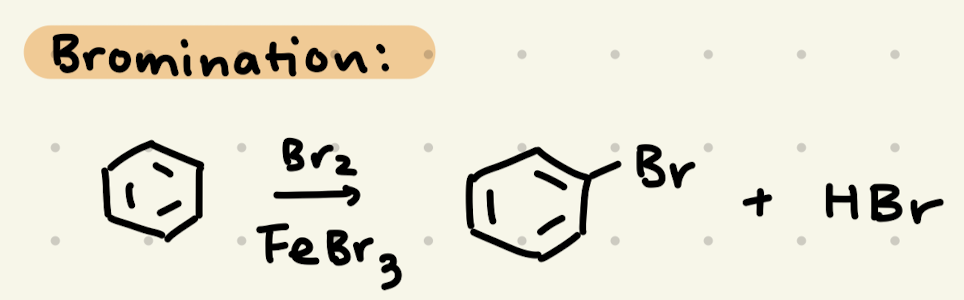
Br2, FeBr
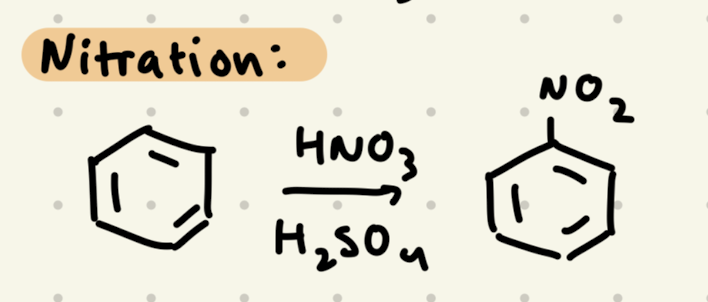
HNO3, H2SO4
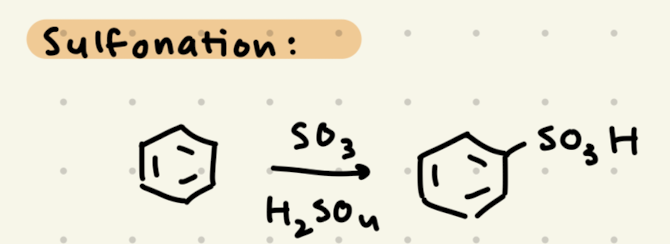
SO3, H2SO4
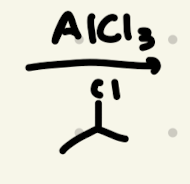
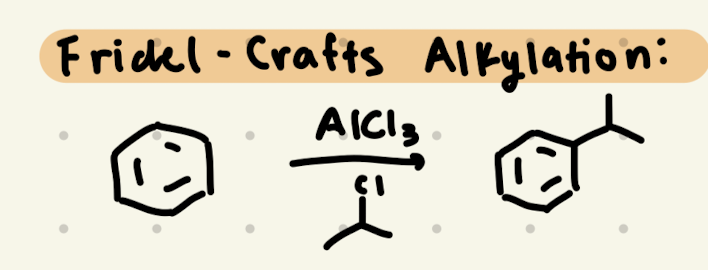
→ we need to do carbocation rearrangements!
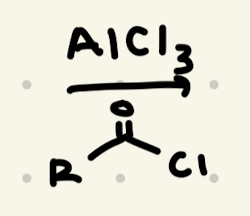
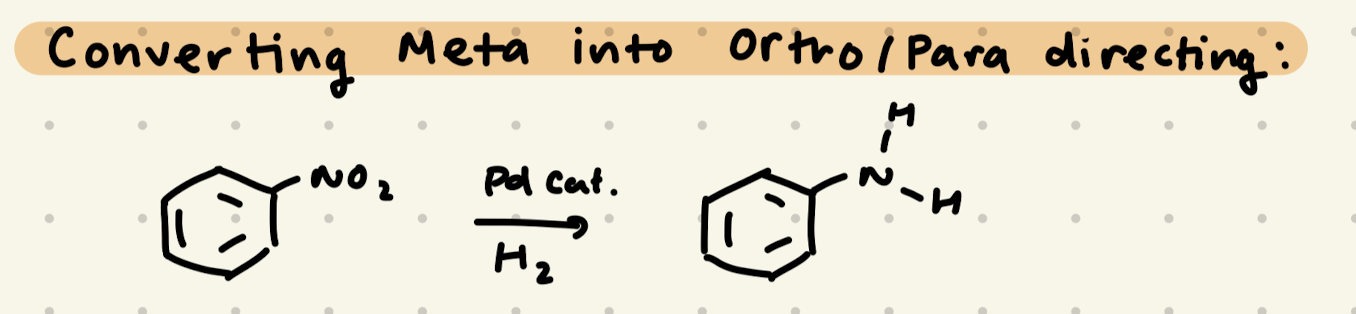
Positions of Aromatic Rings, Ortho/Para Directors, Meta Directors
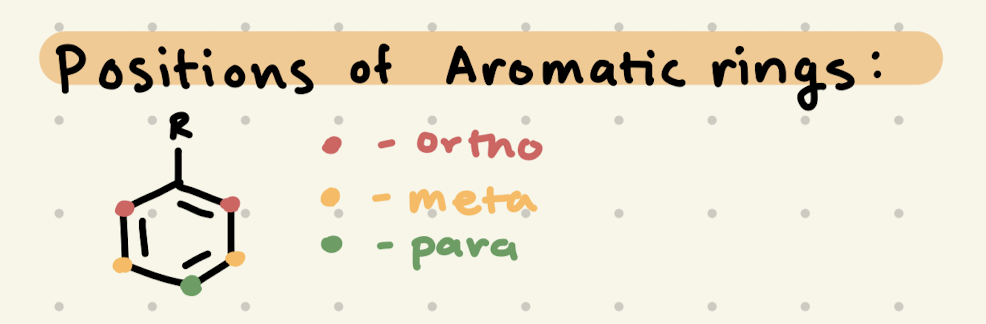
Electron Donating Groups are Ortho/Para directors
Electron Withdrawing Groups are Meta directors
cat. Pd, H2