alkenes
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
31 Terms
alkanes
Unsaturated hydrocarbons containing a C=C, can be straight chains branched chains or rings
Formulas
Straight chains or branched chain allenes with one C=C double bond have the general formula Cn H2n
What does the C=C bond comprise
A pi bond: bond formed from the sideways overlap of adjacent p orbitals above and below the bonding carbon atoms preventing rotation about the C=C
What does the C=C bond comprise (2)
A sigma bond formed from the overlap of orbitals directly between bonding atoms
Shaped and bond angle around each carbon in the C=C of alkenes in terms of electron pair repulsion
Trigonal planar, 120°
What are stereoisomers
Compound with the same structural formula, molecular formula but with a different arrangement of atoms in space, C=C bon allows alkene to have stereoisomers as well as structural isomers
Type 1 of stereoisomers
E/Z isomerism: as the groups attached to the carbon atoms of the C=C bond have fixed positions and cannot rotate from one side of the double bond to the other side
A molecule will have an E/Z isomer if
There is a C=C bond
There are 2 different groups attached to each carbon atom of the C=C bond
Use of CIP priority rules to identity E and Z stereoisomers
atoms attached to each C atom of the C=C bond are given a priority depending on atomic number , atom with the greater atomic number has the higher priority
Z isomer
Has both higher priority groups on the same side of the C=C bond
E isomer
Has higher priority groups on opposite sides of the C=C bond
Type 2 of stereoisomerism
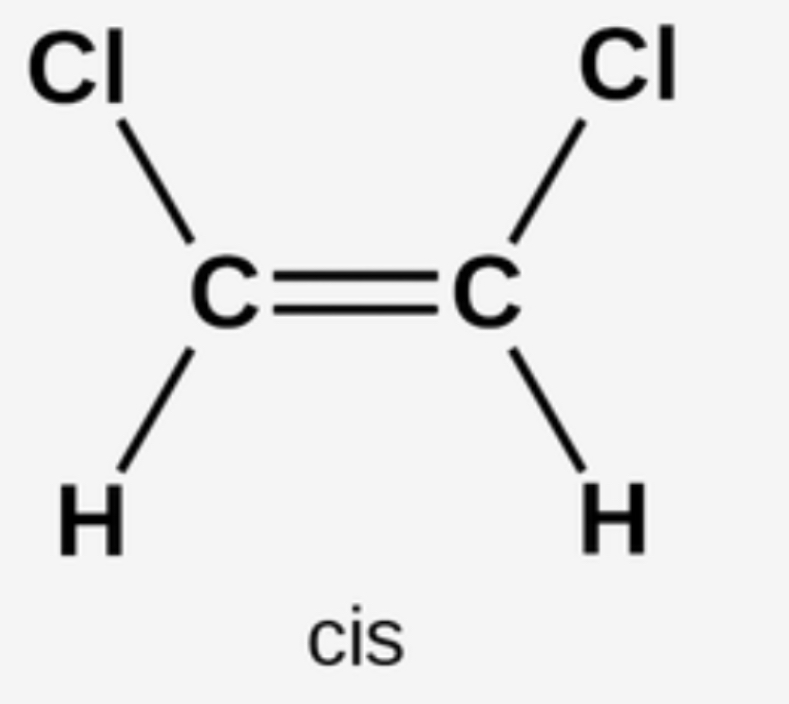
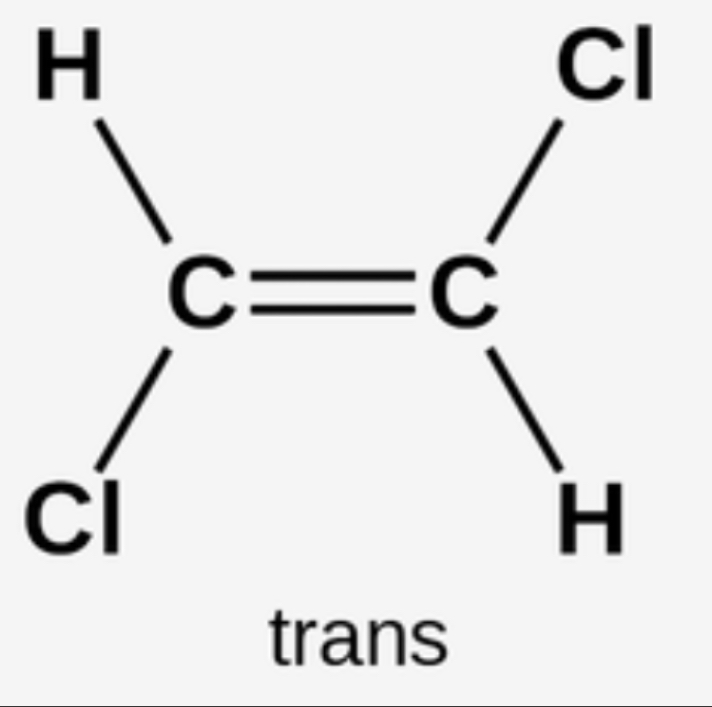
Cis trans isomerism- special case of E/Z isomerism in which two of the substituent groups attached to each carbon atom of the C=C are the same
In a cis isomer
In a trans isomer
Determination of possible E/Z or cis-trans stereoisomers of an organic molecule given its structural formula
Reactivity of alkenes
Much more reactive than alkanes, reactivity of alkenes is caused by the presence of pi bonds
Reason why they are more reactive than alkanes
In the C=C the pi bond has a smaller bond enthalt than the sigma bonds and is broken more easily
Pi bonds
Introduce a region of high electron density above and below the plane of bonding carbon atoms in the C=C bond so electron deficient species (electrophiles) can attack the high electron density of the pi bond causing the alkene to react
addition reactions of alkenes
Undergo many addition reactions to form saturated compounds- a small molecule adds across the double bond causing the pi bonds to break and new sigma bonds form
So during addition to alkenes
the double bond is lost and they become saturated
Addition reactions of alkanes with hydrogen
React with H in the presence of a suitable catalyst such as Ni (nickel) to form alkanes
C2 H4 + H2 —> C2 H6
ethene + hydrogen —→ ethane
Addition reactions of alkenes with halogens
React with halogens to form dihaloalkanes
C2 H4 + Br2 —→ C2 H4 Br2
ethene + bromine —→ dibromoethane
Use of bromine to detect the presence of double C=C bond as a test for unsaturation in a carbon chain
This is because Unsaturated hydrocarbons decolourise orange bromine water
Procedure
Orange bromine water is added to an excess of an organic compound, mixture is shaken
Results
In the presence of a double bond the mixture becomes colourless
With a saturated compound no addition reaction takes place so there is no colour change
Addition reactions of alkenes with hydrogen halides to form haloalkanes
Alkanes react with hydrogen bromide to form bromoalkanes
with symmetrical alkenes such as ethene
With the same groups attached to each carbon atom of the C=C bond, it doesn’t water which way round the HBr adds across the C=C as the product is always the same
C3 H6 + HBr—> C3 H7 Br
with asymmetrical alkenes such as propene
With different groups attached to each carbon atom of the C=C bond abmahnt adds across C=C bond in two different ways to produce 2 different products
CH3 CH=CH2 + HBr —> CH3 CH2 CH2 Br (1-bromopropane)
CH3 CH=CH2 + HBr —> CH3 CHBr CH3 (2-bromopropane)
Addition of a hydrogen halide such as HBr across the double bond of an asymmetrical alkanes
Forms a mixture of products e.g. 2-bromopropane and 1-bromopropane
Primary’s nd secondary carbocation
Formation of 1-bromopropane goes via a primary carbocation , formation of 2-bromopeopane goes via secondary carbocation
2-bromopropane is the major product, 1-bromopropane is a minor product
In mechanisms
The secondary carbocation is more stable than a primary carbocation and is more likely to form