Reaction Types
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
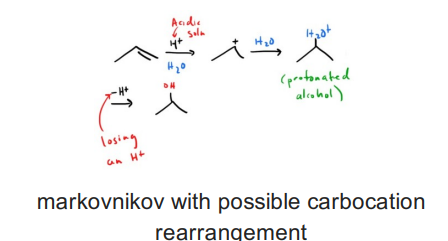
Acid Catalyzed hyrdation of an alkene (acid and water or H3O+)
Addition of HX to an alkene
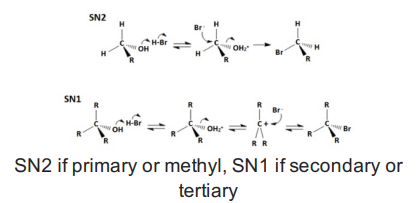
Alcohols to alkyl halides (ROH with HX)
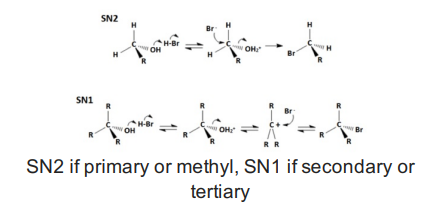
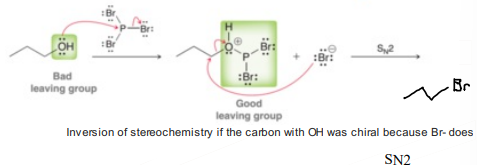
Alcohols with PBr3
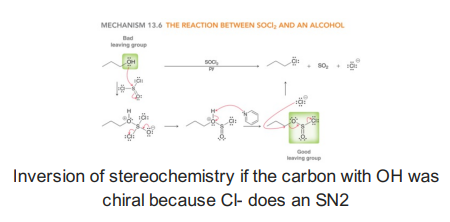
Alcohols with SOCl2
Alkene with CH2I2 and Zn(Cu)
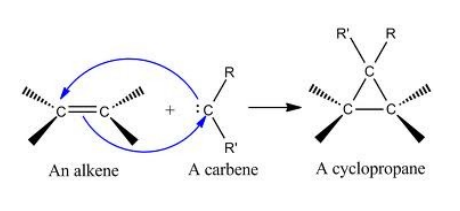
Alkene with CH2N2
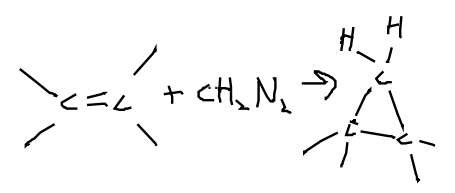
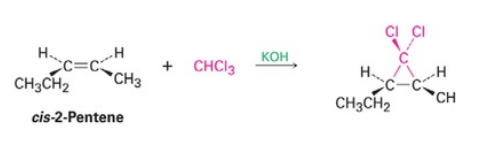
Alkene with CHCl3 and Strong Base
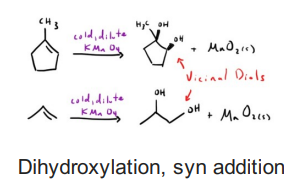
Alkene with KMnO4 (Cold), dilute OH- or 1)OsO4 2)Me2S
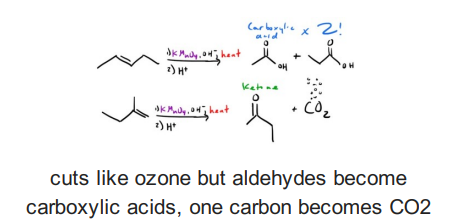
Alkene with KMnO4 (Hot), OH
Alkyne Hydrogenation (3 types)
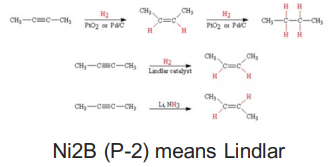
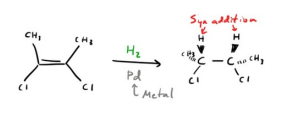
Catalytic Hydrogenation (H2, metal)
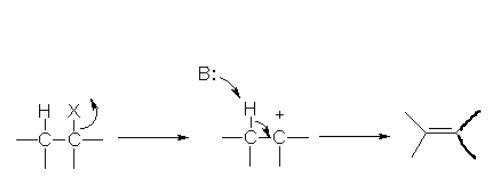
E1 Reaction
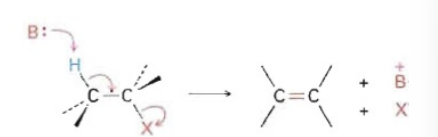
E2 Reaction
Epoxide Reacting with Acid
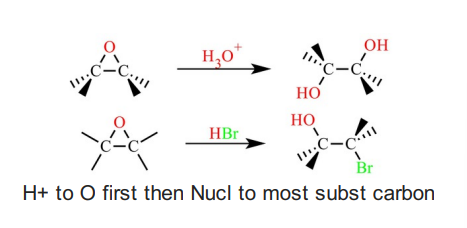
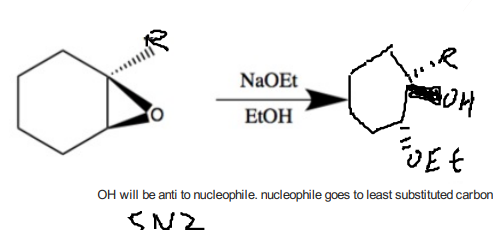
Epoxide reacting with base (good nucleophile)
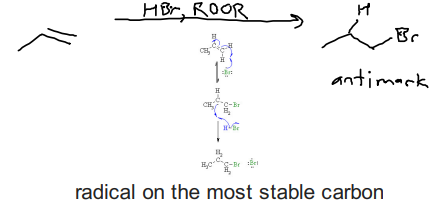
Free Radical Addtion to alkene (HBr, ROOR) Propagation
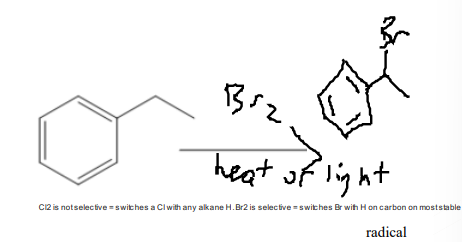
Free Radical Halogenation (Cl2 or Br2 with heat or light)
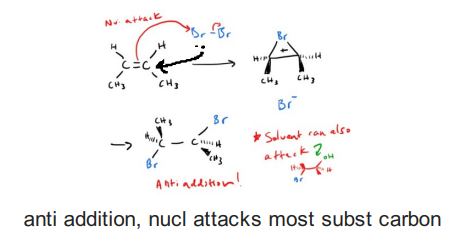
Halogenation of an alkene (X2)
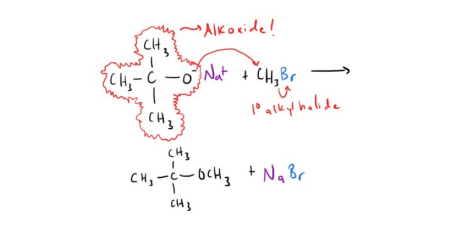
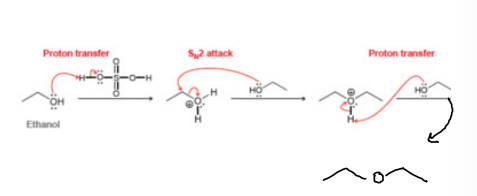
How to Cleave an Ether!
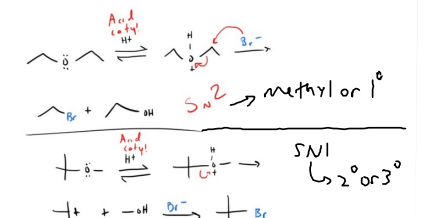
Hydroboration 1)BH, THF 2) H2O2, OH
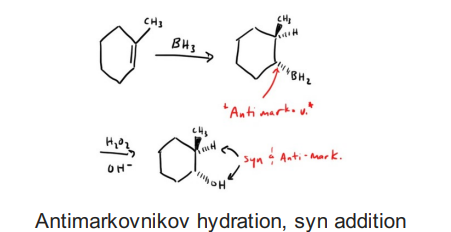
Intermolecular Dehydration of Alcohols (ROH with H2SO4)
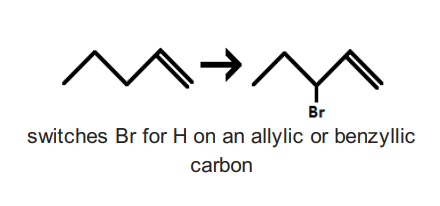
NBS with heat or ROOR
OTs, OTf, OMs
Good leaving groups
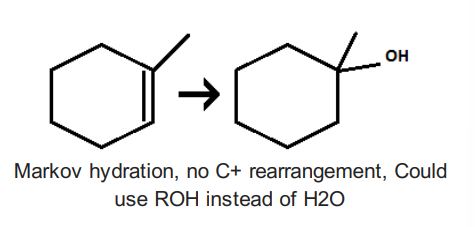
Oxymercuration/Demercuration 1) Hg(OAc)2, H2O 2)NaBH4, OH
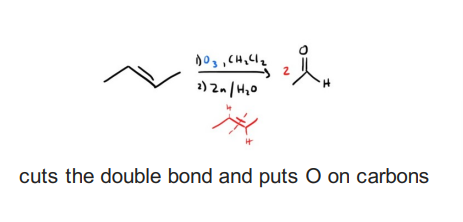
Ozonolysis 1) O3 2) Me2S or Zn
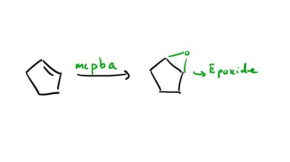
Peroxycarboxylic acids (McPBA) or COOOH
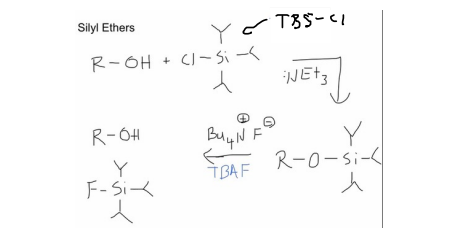
Protecting Groups (ROH with TBSCl)
Sn1 Reaction
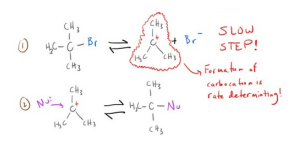
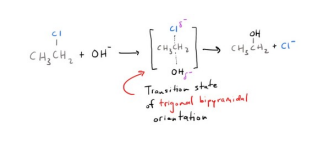
Sn2 Reaction
Strong Bases
N-, O-, C-, H- except: CN-, COO-, N3
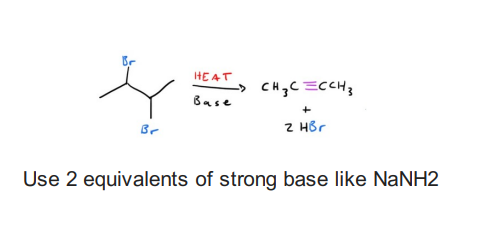
Synthesis of Alkyne starting from dihalide
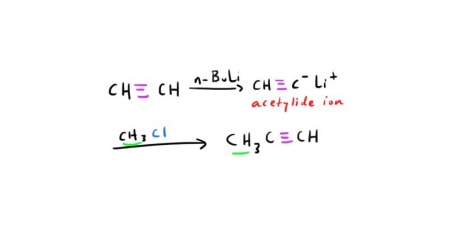
Synthesis of Alkyne using acytelide ion
William Ether Synthesis RO- with R-LG