REQUIRED PRACTICALS: PAPER 1
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
7 Terms
Require practical 1: making salts

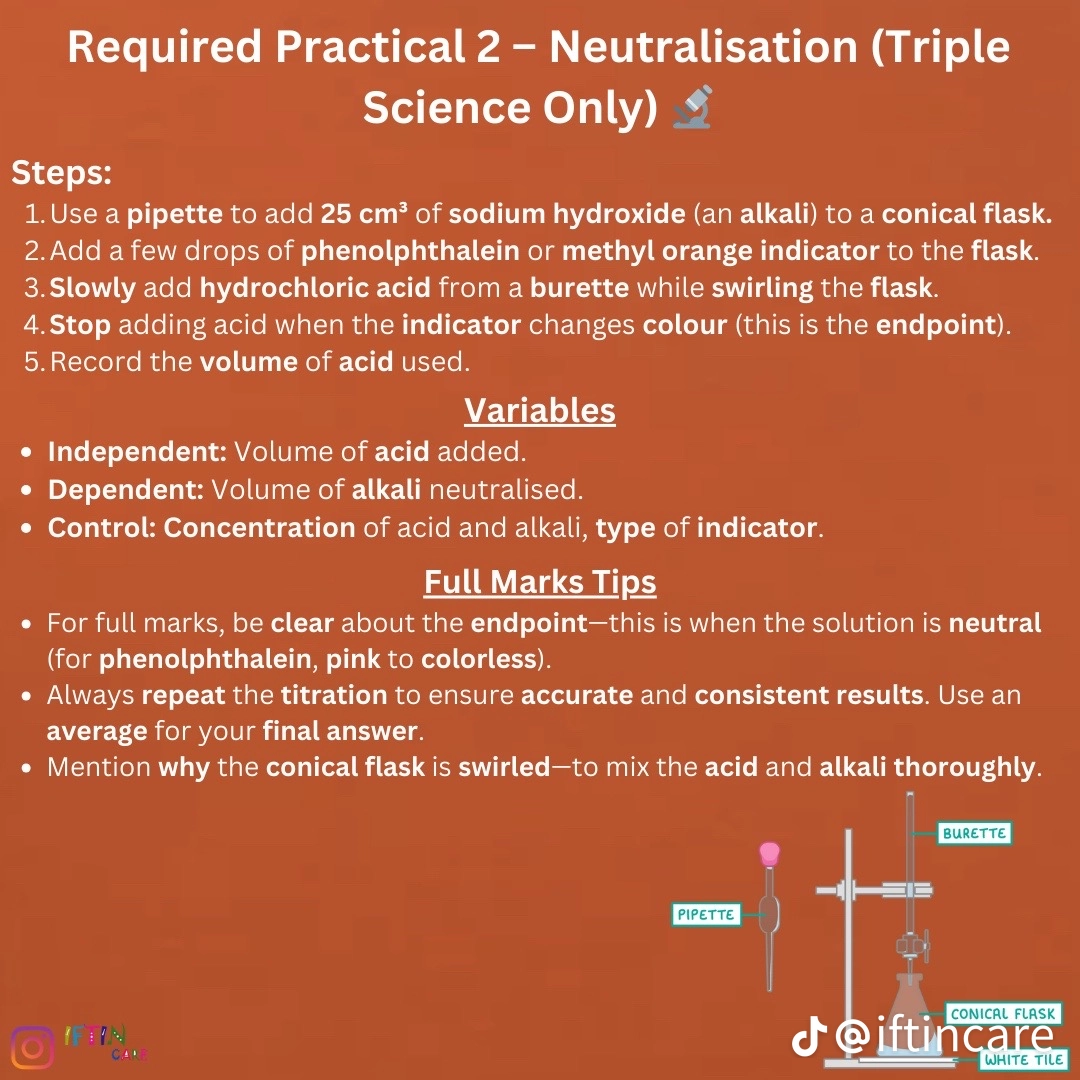
Required practical 2: neutralization
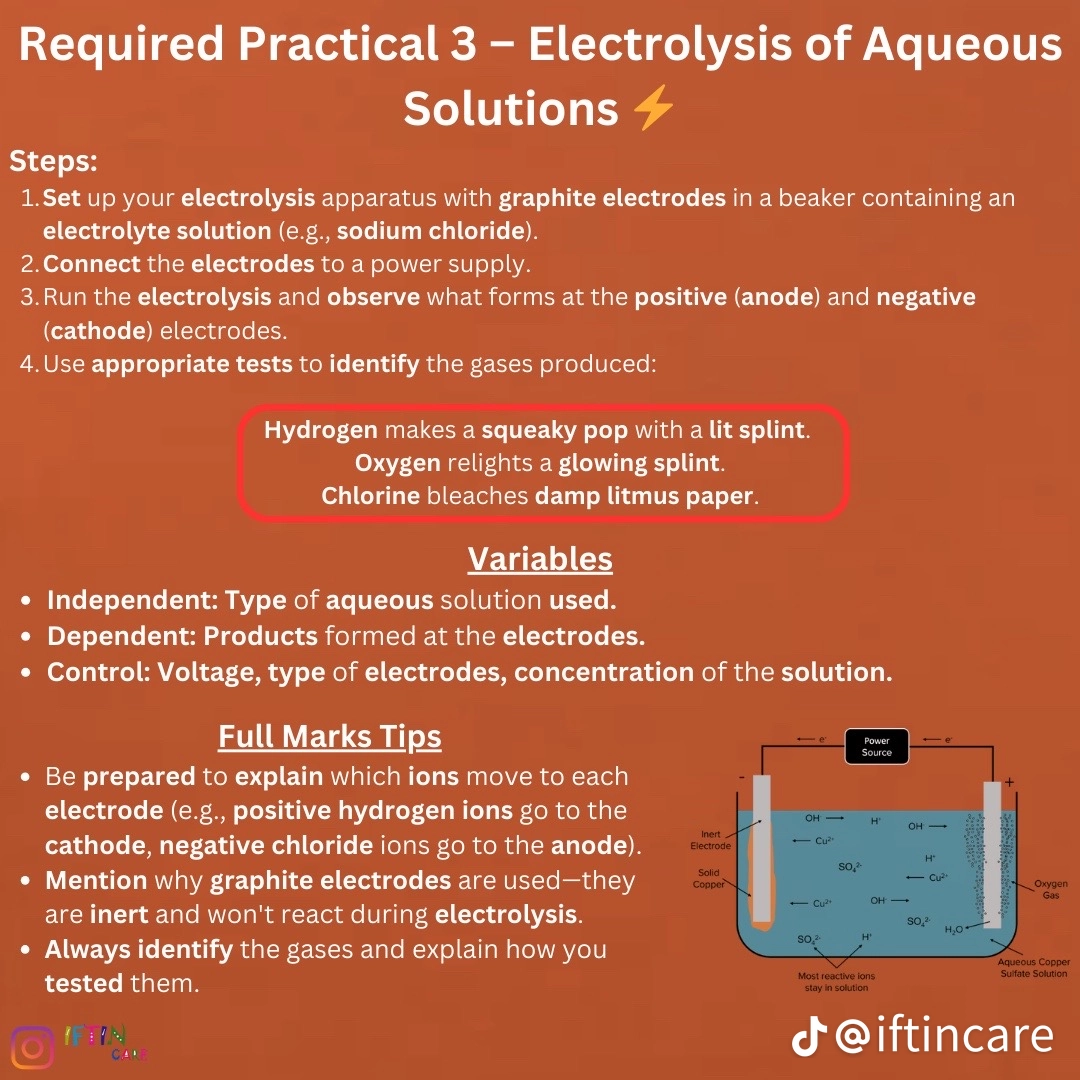
Required practucal 3: electrolysis of aqueous solution.
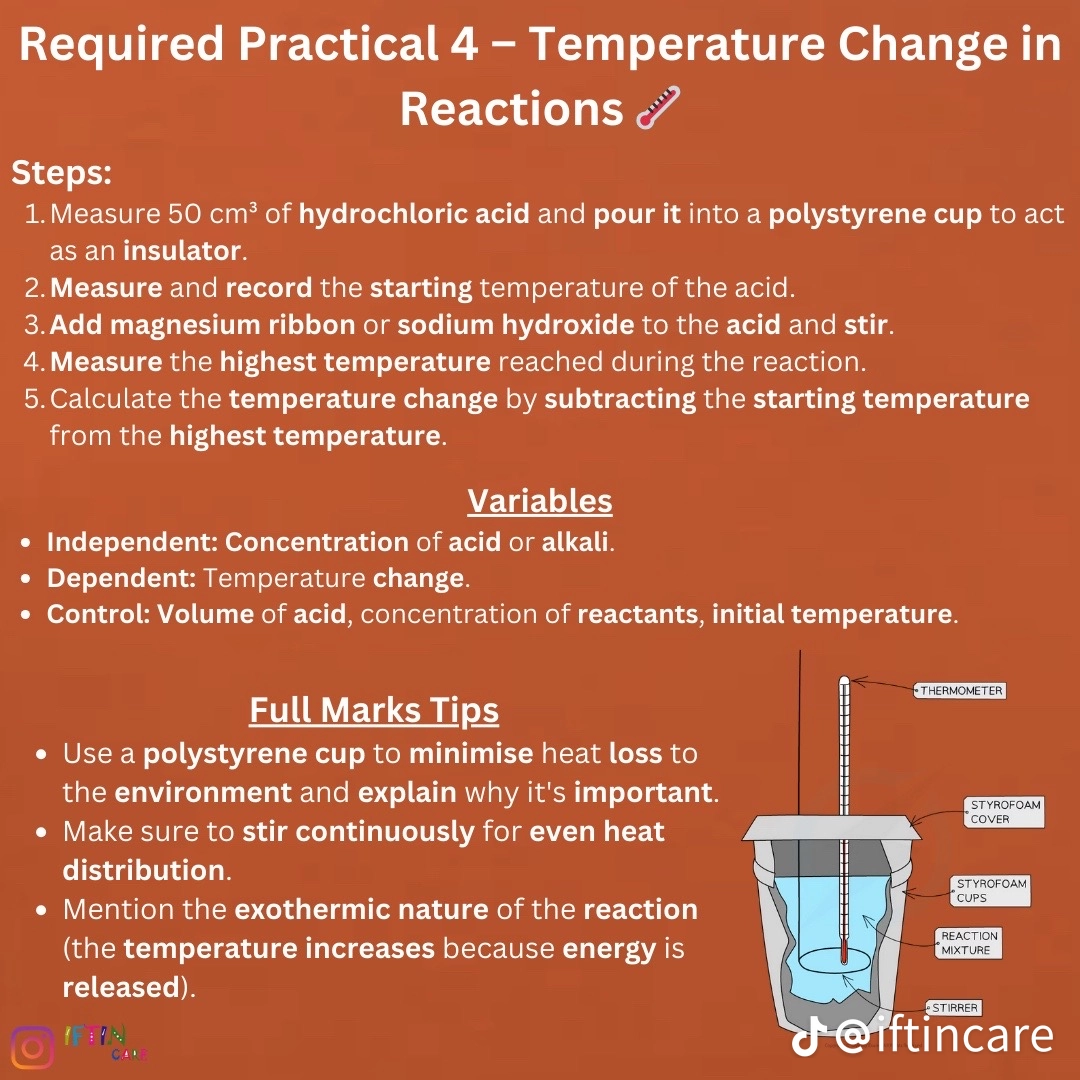
Required practical 4; temperature change in reactions
Titration
use a pipette to transfer 25cm3 of sodium hydroxide solution (base) into a conical flask (helps reduce splashing)
Add five drops of indicator such as methyl orange to the alkali in conical flask
Place conical flask on a white tile so colour change can be seen more clearly
Fill a burette with acid
Add acid to the alkali until the solution is neutral we need to add just enough acid to see this colour change. Once we start to see a colour change we now add the acid drop by drop until the solution is neutral. It is important to swirl so acid and base mix ( orange to red)
Read volume of acid added from the burette