QC1 LEC PRE FI: Precipitation Method
1/92
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
93 Terms
Precipitate
Occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a _______
Precipitation Method
Require the formation of relatively insoluble substances or precipitates
Cessation of Precipitation
Turbidity
Determination of Endpoint can be done in:
_____ or the appearance of a _____
Internal Indicators
Determination of Endpoint can be done in:
Use of _____
Potentiometric/Amperometric
Determination of Endpoint can be done in:
Instrumental Methods: ____/____
Ferric Ammonium Sulfate
Potassium Chromate
Adsorption Indicators
Indicators
Dichlororfluorescein (DCF)
Eosin Y TS
Tetrabromophenolphthalein Ethyl Ester (TEE) TS
Adsorption Indicators
Ferric Ammonium Sulfate
What indicator has the usage of Direct and Residual Titrations with Standard Ammonium Thiocyanate Solution
Ferric Ammonium Sulfate
What indicator is being described:
8g of NH₄Fe(SO₄)₂·12H₂O (Ammonium Iron Sulfate Dodecahydrate) in sufficient water to make 100mL
100mL
In Ferric Ammonium Sulfate,
8g of NH₄Fe(SO₄)₂·12H₂O (Ammonium Iron Sulfate Dodecahydrate) in sufficient water to make _____
Ammonium Iron Sulfate Dodecahydrate
NH₄Fe(SO₄)₂·12H₂O
White
In Ferric Ammonium Sulfate,
Thiocyanate reacts with Hg/Ag to produce a ____ ppt
Red
In Ferric Ammonium Sulfate,
Once all Hg/Ag have precipitated, thiocyanate ions react with indicator, it forms ____ (endpoint) Ferric Thiocyanate
Potassium Chromate
What indicator is being described:
10g of K₂CrO₄ in sufficient water to make 100mL solution
White
In Potassium Chromate,
Forms ____ ppt with AgCl at start of reaction
Red
In Potassium Chromate,
Forms ___ ppt of Ag Chromate - marks endpoint
AgCl
Potassium Chromate has coprecipitation with ___
Adsorption Indicators
Are weak organic acids that vary in strength
Dichlororfluorescein (DCF) TS
What Adsorption Indicator is being described:
100mg of DCF in 60mL ROH → add 2.5mL of 0.1N NaOH → mix and dilute with water to 100mL
Eosin Y TS
What Adsorption Indicator is being described:
500mg of Eosin Y in 100mL of water
Tetrabromophenolphthalein Ethyl Ester (TEE) TS
What Adsorption Indicator is being described:
100mg of TEE in 90mL of glacial HAc and dilute with glacial HAc to 100mL
Fresh Solution
Tetrabromophenolphthalein Ethyl Ester (TEE) TS is prepared as ____
Endpoint
_____ is indicated by Abrupt change of color of the silver halide ppt due to absorbed indicator anions
Diffuse-light condition
Endpoint is best seen at _____
0.1N Silver Nitrate (AgNO₃)
0.1N Ammonium Thiocyanate (NH₄SCN)
Standard Solutions
slow
In 0.1N Silver Nitrate,
Diluted HCL is a ___ precipitant
Hydrochloric Acid
In 0.1N Silver Nitrate,
This aids in the precipitation with large particles of silver
Purple
In 0.1N Silver Nitrate,
Free silver is produced _____ when exposed to light
0.1N Ammonium Thiocyanate (NH₄SCN)
In preparation & standardization,
A secondary standard; Deliquescent
0.1N Silver Nitrate (30mL) + HNO₃ (2mL)
Analyte of 0.1Ammonium Thiocyanate
Ferric Ammonium Sulfate (2mL)
Indicator of 0.1N Ammonium Thiocyanate
Permanent Reddish-Brown
Endpoint of 0.1N Ammonium Thiocyanate
Nitric Acid (HNO₃)
In Ammonium Thiocyanate
This prevents hydrolysis of FeNH₄(SCN)₂
Calculation for Normality
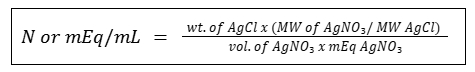
Direct Titration
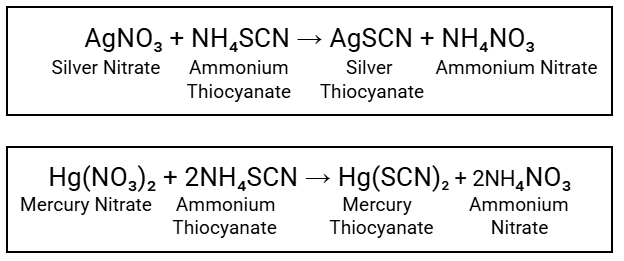
Direct Titration/Residual Titration:
Used for compounds that are readily convertible into soluble salts
Silver & Mercury
Example of Direct Titration
Ammonium Thiocyanate Solution (NH₄SCN
Titrant in Direct Titration
Ferric Ammonium Sulfate
Indicator in Direct Titration
Red ppt of Ferric Thiocyanate
Endpoint in Direct Titration
Direct Titration
acidified
In Direct Titration,
Solution must be ___ with HNO₃
In direct titration, acidifying HNO₃ prevents ____ of ferric salts in neutral solution
Chlorides
In direct titration, ___ must be absent
Since chlorides of silver and mercury are more soluble than the respective thiocyanates
In direct titration, why is chlorides must be absent?
Assay of Phenylmercuric Nitrate
In direct titration, what assay is being described:
Used for mercury content determination
0.1N Ammonium Thiocyanate
Titrant of Phenylmercuric Nitrate
Ferric Ammonium Sulfate
Indicaror of Phenylmercuric Nitrate
62.75% - 63.50% Hg
NF requirement of Phenylmercuric Nitrate
Assay of Phenylmercuric Nitrate
In direct titration, what assay is being described:
Antiseptic and Antifungal agent; Preservative in Pharmaceutical 0.0025%
Sodium Lauryl Sulfate
A cleaning agent
Na Dodecyl Sulfate
Assay of Sodium Lauryl Sulfate, AKA?
0.1N Silver Nitrate
Titrant of Assay of Sodium Lauryl Sulfate
Potassium Chromate
Indicator of Assay of Sodium Lauryl Sulfate
Assay of Sodium Lauryl Sulfate
In direct titration, this has coprecipitation of Ag Chromate and Ag Chloride
Assay of Iopanoic Acid Tablets
3-amino-α-ethyl-2,4,6-triiodobenzenepropanoic acid
Assay of Iopanoic Acid Tablets
In direct titration,
This assay is used for iodine ion determination
0.05N Silver Nitrate
Titrant of Assay of Iopanoic Acid Tablets
Glacial HAc + TEE TS
Indicator of Assay of Iopanoic Acid Tablets
Yellow ppt changes to green
Endpoint of Assay of Iopanoic Acid Tablets
0.1N Silver Nitrate
Titrant of Benzyltrimethylammonium Chloride
Dichlorofluorescein
Indicator of Benzyltrimethylammonium Chloride
Volhard Method
NaCl Purity Determination
Assay of NaCl
Assay for Iodide content of Povidone-Iodine (Betadine)
Assay of Theophylline
Residual Titration
Jacob Volhard
Who discovered the Volhard Method
Volhard Method
NaCl Purity determination can also be determined by ___
Absent
Sodium Chloride Purity Determination can be determined by Volhard Method provided by, substances which give precipitates with silver nitrate are ____
0.1N Silver Nitrate
Titrant 1 of Assay of NaCl (added in excess)
0.1N Ammonium Thiocyanate
Titrant 2 of Assay of NaCl
Ferric Ammonium Sulfate
Indicator of Assay of NaCl
Nitric Acid
In residual titration, assay of NaCl
This Acid is added to prevent ppt of silver with other halides
In residual titration, assay of NaCl
This is added to form a film over precipitated AgCl particles
100.5%
In residual titration, assay of NaCl has a USP req of ____
%Purity of NaCl Formula
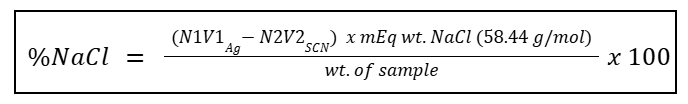
Betadine
Assay for Iodide Content of Povidone-Iodine or ___
Assay for Iodide Content of Povidone-Iodine (Betadine)
In residual titration, what assay is described:
Reaction must produce reduced iodine ion
0.1N Silver Nitrate
Titrant 1 of Assay for Iodide Content of Povidone-Iodine (Betadine)
Ferric Ammonium Sulfate
Indicator for Assay for Iodide Content of Povidone-Iodine (Betadine)
0.1N Ammonium Thiocyanate
Titrant 2 Assay for Iodide Content of Povidone-Iodine (Betadine)
Sodium Bisulfite T. S
In residual titration, Assay for Iodide Content of Povidone-Iodine (Betadine)
This makes iodine colorless (reduced iodine)
Nitric Acid
In residual titration, Assay for Iodide Content of Povidone-Iodine (Betadine)
Prevent hydrolysis of ferric alum and ppt of silver as carbonate, phosphate, etc.
6.6%
In residual titration, what is the USP requirement of Assay for Iodide Content of Povidone-Iodine (Betadine)
8-chlorotheophylline
Assay Of Theophylline or AKA ___
Aminophylline
Assay Of Theophylline, Official Prep ____
0.1N Silver Nitrate
Titrant 1 of Assay of Theophylline
Ferric Ammonium Sulfate
Indicator of Assay of Theophylline
0.1N Ammonium Thiocyanate
Titrant 2 of Assay Of Theophylline
Nitric Acid
In Assay of Theophylline, what is used to acidify silver nitrate
Sodium Tetraphenylboron Titration
Used as titrant to quantitatively precipitate such organic nitrogen compounds as alkaloids, amine, and quaternary salts, as well as ammonium, potassium, and silver ions.
Chloroform
Extratcion Indicator of Sodium Tetraphenylboron Titration
Bromophenol Blue
In Sodium Tetraphenylboron Titration
Quaternary Compounds + Anionic Dye, such as ___
Insoluble Compound
In Sodium Tetraphenylboron Titration
Colored Complex + Titrant Na(C₆H₅)₄B =____
colorless
Endpoint of Sodium Tetraphenylboron Titration
Determined when the chloroform layer becomes _____ due to the liberation of nitrogen from dye complex to precipitate with the tetraphenylboron
Sodium Tetraphenylboron Titration
Offers advantages than tedious residual methods
Its uses alkaloidal precipitants such as Potassium ferric cyanide and K₂CrO₄ (alkaloidal ppts)
Benzethonium Chloride
Cetylpyridinium Chloride
In Sodium Tetraphenylboron Titration, drugs that undergo this reaction)