Chapter 10 - Atomic Structure and Atomic Spectra (copy)
0.0(0)
0.0(0)
Card Sorting
1/42
Earn XP
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
1
New cards
Electronic structure of an atom
The arrangement of electrons around a nucleus
2
New cards
Hydrogenic atom
A one-electron atom or ion of general atomic number Z
3
New cards
Many-electron atom (Polyelectronic atom)
An atom or ion with more than one electron
4
New cards
Rydberg constant for the hydrogen atom
RH = 109677 cm^-1
5
New cards
Ritz combination principle
States that the wavenumber of any spectral line is the difference between two terms
6
New cards
Coulomb potential energy of an electron in a hydrogenic atom
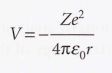
7
New cards
Radial wave equation
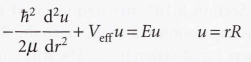
8
New cards
Bohr radius
It is called like this because the same quantity appeared in Bohr's early model of the hydrogen atom as the radius of the electron orbit of lowest energy
9
New cards
Atomic orbital
A one-electron wavefunction for an electron in an atom
10
New cards
Principal quantum number (n)
It can take the values n = 1, 2, 3, , and determines the energy of the electron
11
New cards
Bound states of the atom
Where the energy of the atom is lower than that of the infinitely separated, stationary electron and nucleus
12
New cards
Unbound states of the electron
The states to which an electron is raised when it is ejected from the atom by a high-energy collision or photon
13
New cards
Ionization energy (I)
The minimum energy required to remove an electron from the ground state, the state of lowest energy, of one of its atoms
14
New cards
Shell
All the orbitals of a given value of n
15
New cards
Subshell
The orbitals with the same value of n but different values of l
16
New cards
Angular momentum quantum number (l)
Depends on the principal quantum number
17
New cards
Radial distribution function P(r)
A probability density in the sense that, when it is multiplied by dr, it gives the probability of finding the electron anywhere between the two walls of a spherical shell of thickness dr at the radius r
18
New cards
Transition
When an electron moves from a higher energy orbital to a lower energy orbital
19
New cards
Selection rule
A statement about which transitions are allowed
20
New cards
Grotrian diagram
It summarizes the energies of the states and the transitions between the selection rules and atomic energy levels
21
New cards
Orbital approximation
Its when we suppose that a reasonable first approximation to this exact wavefunction is obtained by thinking of each electron as occupying its "own" orbital
22
New cards
Pauli exclusion principle
No more than two electrons may occupy any given orbital, and if two do occupy one orbital, then their spins must be paired
23
New cards
Pauli principle
When the labels of any two identical fermions are exchanged, the total wavefunction changes sign; when the labels of any two identical bosons are exchanged, the total wavefunction retains the same sign
24
New cards
Slater determinant
Any acceptable wavefunction for a closed-shell species
25
New cards
Valence electrons
The electrons in the outermost shell of an atom in its ground state
26
New cards
Building-up principle
It says that the order of occupation is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s
27
New cards
Hund's maximum multiplicity rule
An atom in its ground state adopts a configuration with the greatest number of unpaired electrons
28
New cards
First ionization energy
The minimum energy necessary to remove an electron from a many-electron atom in the gas phase
29
New cards
Second ionization energy
The minimum energy needed to remove a second electron from the cation
30
New cards
Electron affinity
The energy released when an electron attaches to a gas-phase atom
31
New cards
Potential energy of the electrons
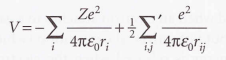
32
New cards
Quantum defect
An empirical quantity
33
New cards
Rydberg states
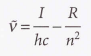
34
New cards
Singlet
The paired-spin arrangement
35
New cards
Triplet
The resulting state when the angular momenta of two parallel spins add together to give a nonzero total spin
36
New cards
Spin-orbit coupling
The interaction of the spin magnetic moment with the magnetic field arising from the orbital angular momentum
37
New cards
Spin-orbit coupling constant
The dependence of the spin-orbit interaction on the value of j
38
New cards
Fine structure of a spectrum
The structure in a spectrum due to spin-orbit coupling
39
New cards
A term symbol gives three pieces of information
* The letter (P or D in the examples) indicates the total orbital angular momentum quantum number, L.
* The left superscript in the term symbol (the 2 in P^2) gives the multiplicity of the term.
* The right subscript on the term symbol (the 3/2 in P_3/2) is the value of the total angular momentum quantum number, J.
* The left superscript in the term symbol (the 2 in P^2) gives the multiplicity of the term.
* The right subscript on the term symbol (the 3/2 in P_3/2) is the value of the total angular momentum quantum number, J.
40
New cards
Total orbital angular momentum quantum number (L)
It tells us the magnitude of the angular momentum
41
New cards
Multiplicity of a term
The value of 2S + 1, where S is the total spin quantum number
42
New cards
Clebsch-Gordan series
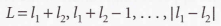
43
New cards
Russell-Saunders coupling
This scheme is based on the view that, if the spin-orbit coupling is weak, then it is effective only when all the orbital momenta are operating cooperatively