Extraction and uses of metals — TRIPLE CONTENT
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
Mineral
Found in the Earth’s crust and are rich in a metal (or metal ions)
Is a specific metal compound
Ore
Is a rock with a high amount of the mineral/rich in the metal compound
It has to be rich enough in the mineral to be worth extracting to be considered ores
All ores are minerals but not all minerals are ores. Ores are specific minerals that contain high concentrations of valuable elements like metals, making them economically viable to extract and use
Where are most metals found?
What about the other type of metals + which are in which category?
Most metals are found in the Earth’s crust combined with other elements in ores (a rock)
A very few unreactive metals like gold are found native/in the Earth’s crust as the uncombined element
What does the extraction of a metal typically involve? Explain how the method of extraction of a metal is related to its position in the reactivity series
Usually removing oxygen from the metal oxides.
If the ore contains a metal which is below carbon in the reactivity series, then the metal is extracted by heating with a reducing agent like carbon (cheap) in a displacement reaction/redox.
Because carbon is more reactive, it will displace the metal from its oxide/carbon is reducing agent so it reduces (gains oxygen) Fe2O3.
Is the ore contains a metal which is above carbon in the reactivity series, then electrolysis is used to extract the metal.
Large amounts of electricity needed = expensive
what are the two main economic factors to take into account for the methods of extraction of metals?
Cost of energy
Cost of the reducing agent
Extraction of metals above carbon: aluminium
What is the name of its ore? How will we extract aluminium?
Aluminium ore Bauxite is the most abundant in the Earth’s crust
Rocky impurities are removed leaving the compound aluminium oxide (Al2O3)
Molten bauxite is dissolved in molten cryolite so Al³+ ions and O²- ions are free to move
Electrolysis is used to separate aluminium from oxygen as Al is more reactive than carbon, so carbon wouldn’t be able to displace aluminium from its compound
During electrolysis to extract aluminium, what happens at the cathode (negative electrode)?
Electrolysis
Negative cathode: positive aluminium ions (Al³+) are attracted to the cathode. Ions gain electrons (reduction) to become aluminium atoms
Al³ + 3e- → Al Reduction
During electrolysis to extract aluminium, what happens at the anode (positive electrode)?
Positive anode: negative oxygen ions (O²-) are attracted to the anode. Ions lose electrons (oxidation) to become oxygen atoms. The atoms join together to form O2 molecules
2O² → O2 + 4e- Oxidation
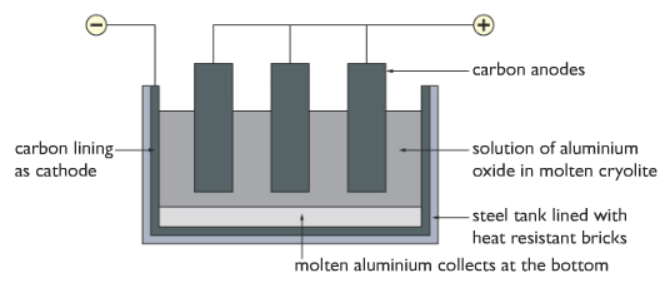
During extraction of aluminium by electrolysis why is the negative cathode at the base?
To collect the molten aluminium.
Positive Al³+ ions are attracted to the negative cathode, gain electrons and become neutral aluminium atoms, which then melt and sink to the bottom to the cathode. We turn circuit off and drain molten Al.
What is the problem of using electrolysis to extract metals? What solution is there?
It is very expensive as it uses a lot of energy to reach the temperature needed.
Aluminium oxide has a very high melting point (melts at 2050°C). We could add a compound called cryolite to lower the melting point to 850°C. This is bc it would give us an impure substance (a mixture) so it melts over a range of temperatures
Why do anodes need replacing? (2)
Graphite (carbon) is quite inert so it is used in anodes but:
the graphite (carbon) anodes/(positive) electrode may react
with the oxygen formed at the anode from electrolysis (REJECT “from air” IGNORE “from aluminium oxide”) forming carbon dioxide
Carbon + oxygen → carbon dioxide
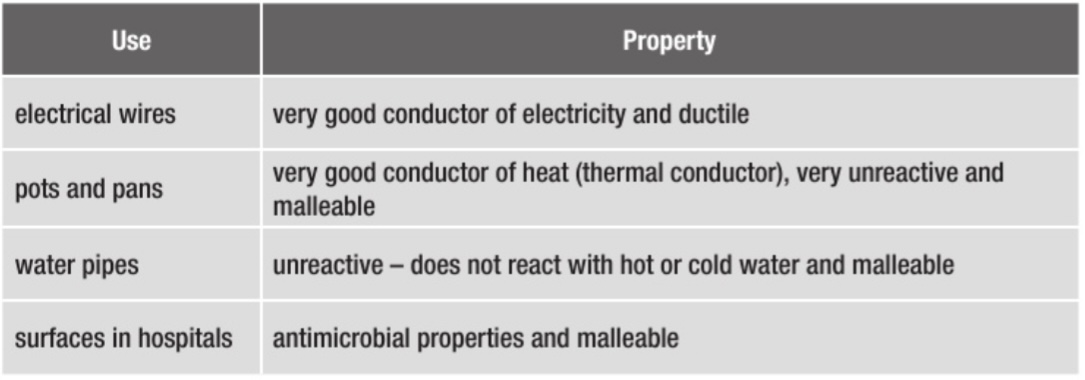
The uses of aluminium depend on…
Pure aluminium is not very strong. It can be strengthened by adding other elements like silicon, copper or magnesium. Uses of Al depend on:
Low density and strength (when alloyed)
Conducts heat and electricity
Can resist corrosion/ it is non-toxic
Malleable (because it is a metal — metallic bonding)
Why does aluminium resist corrosion? Uses of aluminium alloys + give reasons why aluminium is used for each
Aluminium resists corrosion because it has a very thin but very strong layer of aluminium oxide in the surface. This prevents anything else reaching the surface and reacting with it.
Uses include (exam board is obsessed with these)
Pans = conducts heat, resists corrosion
Overhead power cables = conducts electricity, low density, resists corrosion
Aeroplanes = low density, not flamamable
Extraction of zinc + equation
Zinc is often found as ore zinc sulfide (ZnS). To extract it, first it needs roasting (react with O2) to produce zinc oxide — is easier
Equation: 2ZnS (s) + 3O2 (g) → 2ZnO (s) + SO2 (g)
Extraction of metals below carbon: e.g copper + colours
Thermal decomposition (heated) to form copper oxide — copper oxide is more easily reduced by carbon than copper carbonate a common and inexpensive reducing agent
Ore of copper is malachite (CuCO3)
CuCO3 (green) → CuO (black) + CO2
Extraction/redox/displacement
2CuO (s) + C (s) → 2Cu (s) + CO2 (g)
Cu is orange-brown or pink-brown
What is the process of extraction of iron? + equations
This is a continuous process with new raw materials added and products removed all the time due to the time and cost associated with getting the furnace up to temperature
Raw materials: iron ore (hematite), coke (impure form of carbon) and limestone are added into the top of the blast furnace
Raw materials are added from the top of blast furnace
Hot air is blown into the bottom to heat up the inside of the blast furnace. This heat causes the oxygen in the hot air to react with the carbon in coke.
C + O2 → CO2
The reaction between carbon and oxygen is exothermic. This releases more heat inside furnace so this reaction is mainly used to generate heat.
This heat energy released from the above reaction mantains the temperature inside the blast furnace. This high temperature causes carbon dioxide to react with oxygen in the air to produce carbon monoxide. Carbon dioxide has been reduced to carbon monoxide
CO2 + C → 2CO
CO is a powerful reducing agent so it reduces the iron(II)oxide to iron metal. This will melt and collect at the bottom of the furnace.
Fe2O3(s) + 3CO(g) → 2Fe(l) + 3CO2
We use the blast furnace for the redox reaction (carbon will take oxygen away)/also a displacement reaction because carbon is more reactive so will displace iron from its Fe2O3 compound
Iron (III) oxide
By heating with carbon, the reducing agent = Fe2O3 (s) + 3C (s) → 2Fe(l) + 3CO(g)
Other reactions also occur and the main reducing agent is actually carbon monoxide = Fe2O3(s) + 3CO(g) → 2Fe(l) + 3CO2(g)
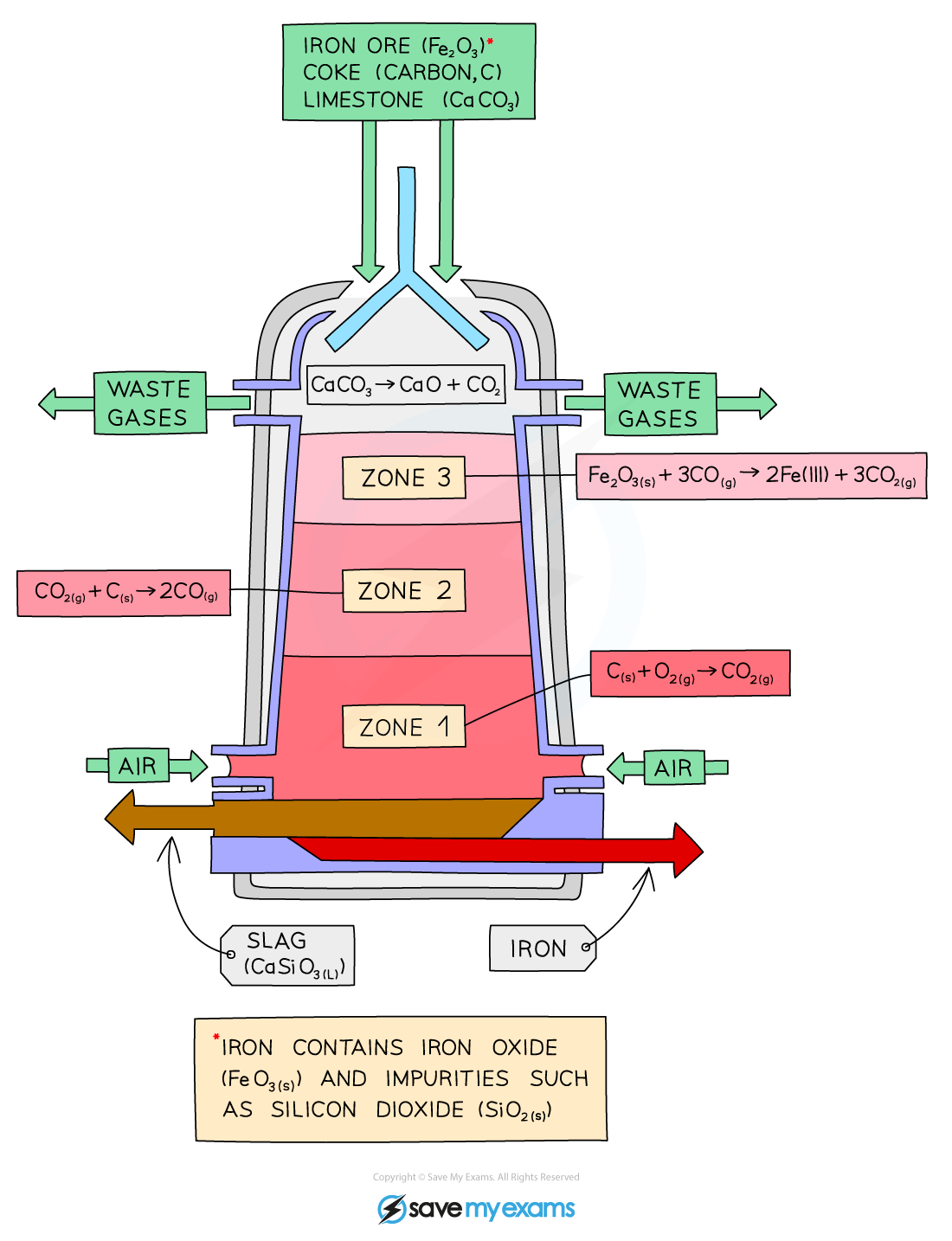
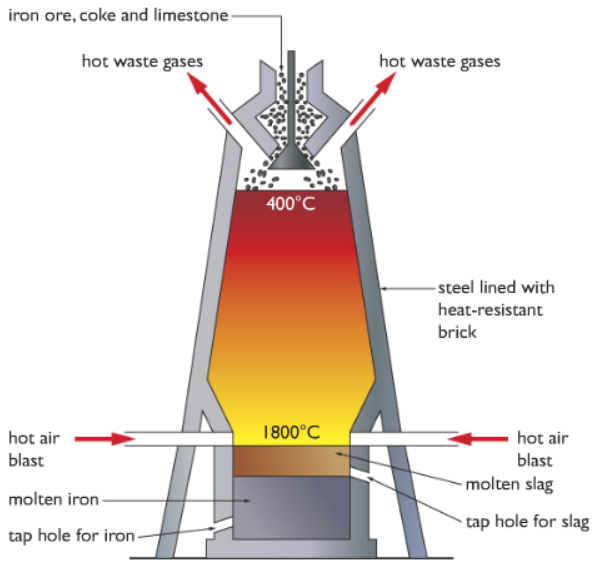
How can we ensure that a minimum amount of heat is lost?
The body of the blast furnace is covered with heat-resistant bricks (insulation)
What is the function of limestone?
Limestone (calcium carbonate, CaCO3) removes the acidic impurities in the ore.
Under the heat of the blast furnace, limestone thermally decomposes to form calcium oxide (and carbon dioxide)
CaCO3 (s) → CaO (s) + CO2 (g)
Calcium oxide then reacts with silicon dioxide to form calcium silicate by neutralisation.
CaO (s) + SiO2 (s) → CaSiO3 (l)
It melts and collects as a molten slag floating above the molten iron, which is recollected separately.
Uses of iron alloys: mild steel
Mild steel
Contains up to 0.25% carbon
Malleable, ductile so can be used in nails, car bodies, ship building, girders and bridges
However, disadvantage of mild steel is that it rusts + denser than aluminium
Some car bodies are made from Al → lighter so less fuel and won’t rust
Uses of iron alloys: high-carbon steel
High carbon steel
Contain about 0.6-1.2% carbon
Harder and more resistant to wear than mild steel but more brittle (not as malleable or ductile)
Used for cutting tools, masonry nails
This alloy also usually contains small amounts of manganese
Uses of iron alloys: stainless steel
Stainless steel
Contains chromium (and often nickel)
Chromium forms a strong oxide layer, protecting the iron. Stainless steel is therefore resistance to corrosion
Uses include kitchen sinks, saucepans, cutlery, gardening tools. Also in brewing (making beer), dairy and chemical industries → corrosion-resistant vessels needed.
What is an alloy?
Is a mixture of a metal with, usually, other metals or carbon.
Why are alloy usually harder than the individual pure metals from which they are made?
In an alloy, different metals/elements have slightly differently sized atoms
This disrupts the regular lattice arrangement of the layers of metal ions
Therefore it makes it more difficult for the layers of ions to slide over each other.
Suggest how increasing the carbon content of steel increases the strength of the steel (2)
- In alloys the regular lattice arrangement of the layers of metal ions is disrupted by carbon atoms
- Therefore it is harder for the layers to slide over each other.
Supongo que the more carbon atoms the harder.
Uses of copper and its alloys + reasons