Formulas & Isomers/Common Terms
1/28
Earn XP
Description and Tags
fundamentals quick study sheets
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
organic chemistry
the study of natural and synthetic materials that have carbon atoms as the key chemical feature. There are more than one million known organic compounds
molecular formula
elemental symbols with subscripts denote the composition of a compound
empirical formula
subscripts denote the relative elemental composition
dash formula
diagram all atoms; show bonds as dashes
bond-line formula
hide H; carbon atoms are depicted as lines, but other atoms are shown explicitly
newman projection
2-d depiction
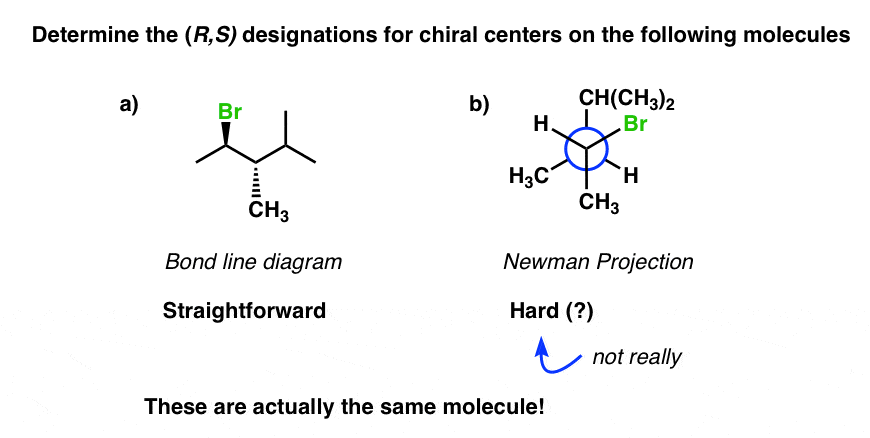
3-dimensional
wedges of sawhorse denote struture
constitutional isomers
different bonding connectivity
ex: rings, bonds, branching, substituent positions
tautomers
easily interconverted structural isomers
chiral
not identical with its mirror image
ex: keto-enol for ketone
achiral
has a plane of symmetry
ex: superimposable on its mirror image
epimers
pair of diastereomers which differ only in the configuration of one atom
aliphatic
non-aromatic
aromatic
benzene ring
conjugation
sequence of alternating double (or triple) and single bonds
dielectric effect
polar solvent stabilizes ion formation
exergonic reactions
chemical reaction that loses energy during the process of the reaction
heterocyclic
non-carbon atom in the ring structure
hydrocarbon
compound of H and C
Ketose
molecule made up of monosaccharide bonded to a ketone
monosaccharide
carbohydrate that cannot be reduced by hydrolysis into another simple sugar
hydrolysis
a chemical process where a molecule is split into two or more smaller molecules by the addition of a water molecule
olefin
alkene
paraffin
alkane
radioisotope
an unstable isotope of an element that spontaneously emits radiation as it decays into a more stable form
(isotope that is radioactive)
saturated
max # of Hs (all C-C single bonds)
tetrose
monosaccharide with all four carbon atoms
unsaturated
at least one C-C multiple bond
zymurgy
the study of the process of fermentation