Inorganic Chem: Energetics definitions
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Exothermic
energy released from the system to the surroundings as heat energy
endothermic
energy absorbed from the surroundings to the systemas heat energy
the system
the chemicals in the reaction mixture
delta H
enthalpy change
enthalpy change
the energy transferred between the system and its surroundings when the change happens at constant pressureand is often measured in joules or calories.
EA
activation energy - the minimum energy required for a chemical reaction to occur.
bond enthalpy
bond energy, which is the energy needed to break one mole of a bond in a gaseous molecule forming gaseous atoms
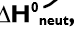
standard enthalpy change of neutralisation
the enthalpy change when an acid and an alkali react together under standard conditions to form one mole of water
standard conditions
a pressure of 100kPa
a specified temperature (if this is not specified it may be taken as 298k)

standard enthalpy of combustion
the enthalpy change when one mole of a substance is burned completely in air or oxygen under standard conditions.
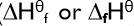
standard enthalpy change of formation
the enthalpy change when one mole of a compound is formed from its elements in their normal physical states under standard conditions.
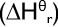
standard enthalpy of reaction
enthalpy change when the molar quantities of reagents shown in a given equation react according to that equation under standard conditions
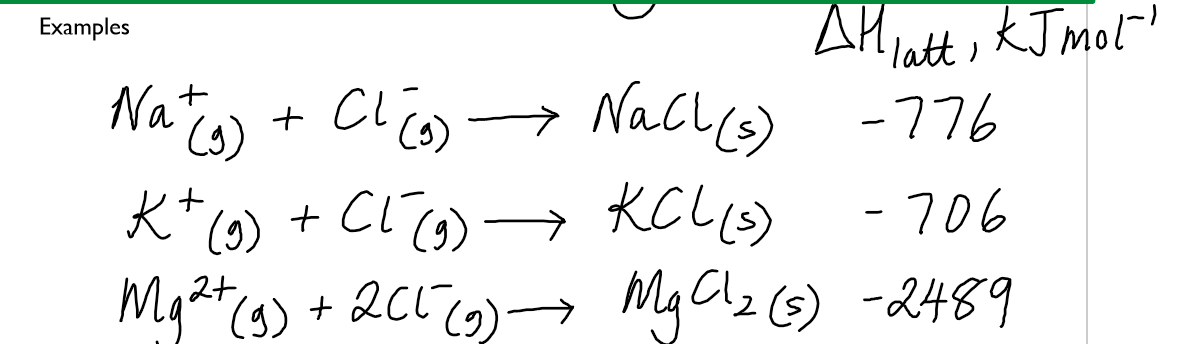
lattice energy
energy released when one mole of a solid ionic lattice is formed from its gaseous ions
1st IE
energy required to remove one electron from each atom in 1 mole of gaseous atoms