Analytical Chemistry: Exam 4
1/177
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
178 Terms
atomic mass unit
This is the mass on an atomic or molecular scale. One of these is equal to 1 Dalton, or 1 g/mol. It is defined based on 1/12 the mass of carbon.
Additionally, one of these units is also equal to 1.66 yg.
mass spectrometry
This analytical technique is used to separate and measure the masses and abundances of analytes, meaning it can be used quantitatively (how much is there) and qualitatively (what is there). In order to use this technique, the analytes must be charged and in the gas phase, which is then injected through a vacuum.
The separation occurs with the use of electric fields, magnetic fields, and radio frequencies. The results are recorded in mass per charge, or m/z.
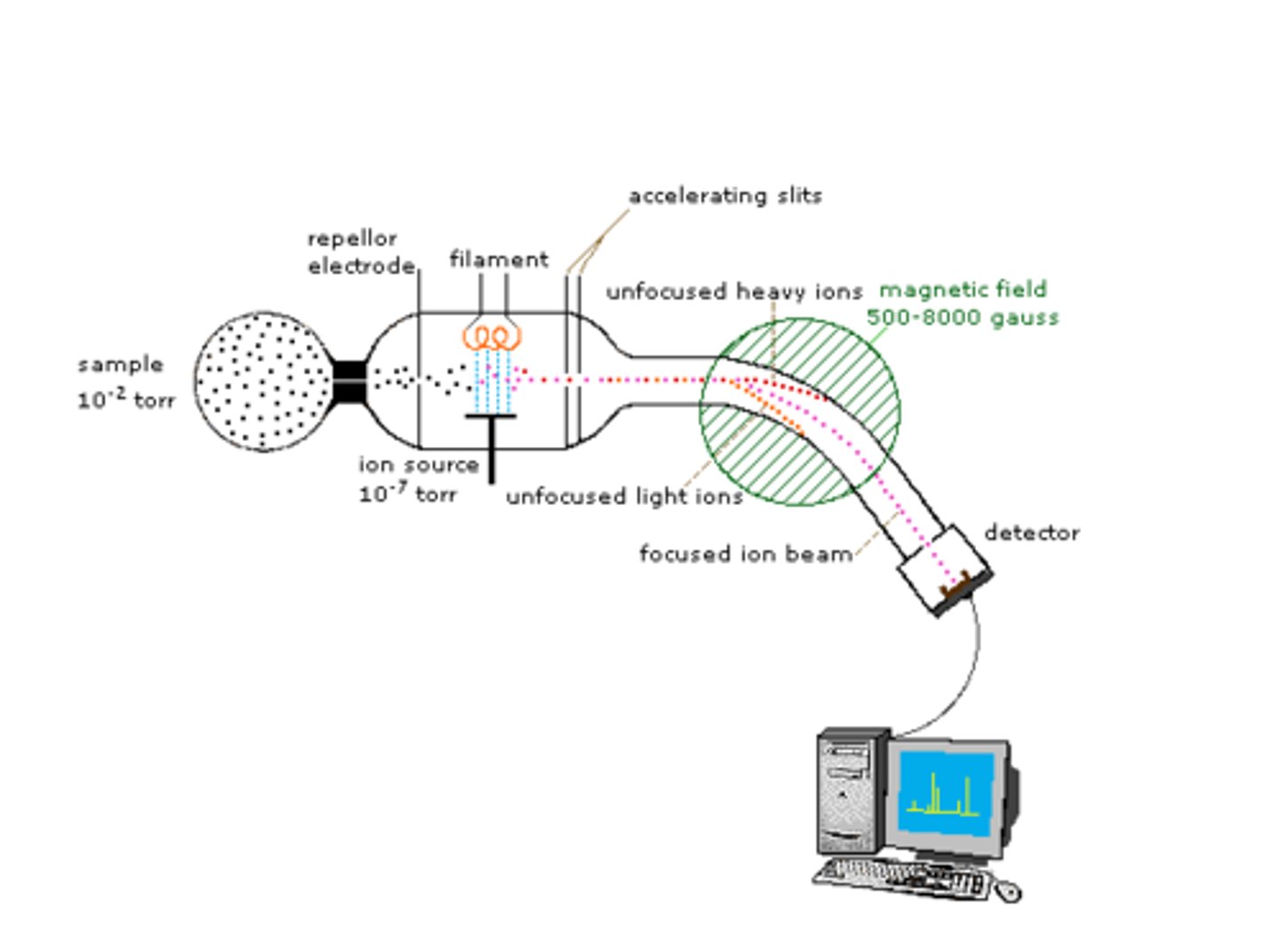
mass per charge
This is the unit the data is recorded with in mass spectrometry. It is the mass of the ion divided by its charge, and is labeled on the x-axis.
total ion current
This is the data plotted on the y-axis, and is the number of ions that strike the detector. The higher this is, the higher the abundance of the ion.
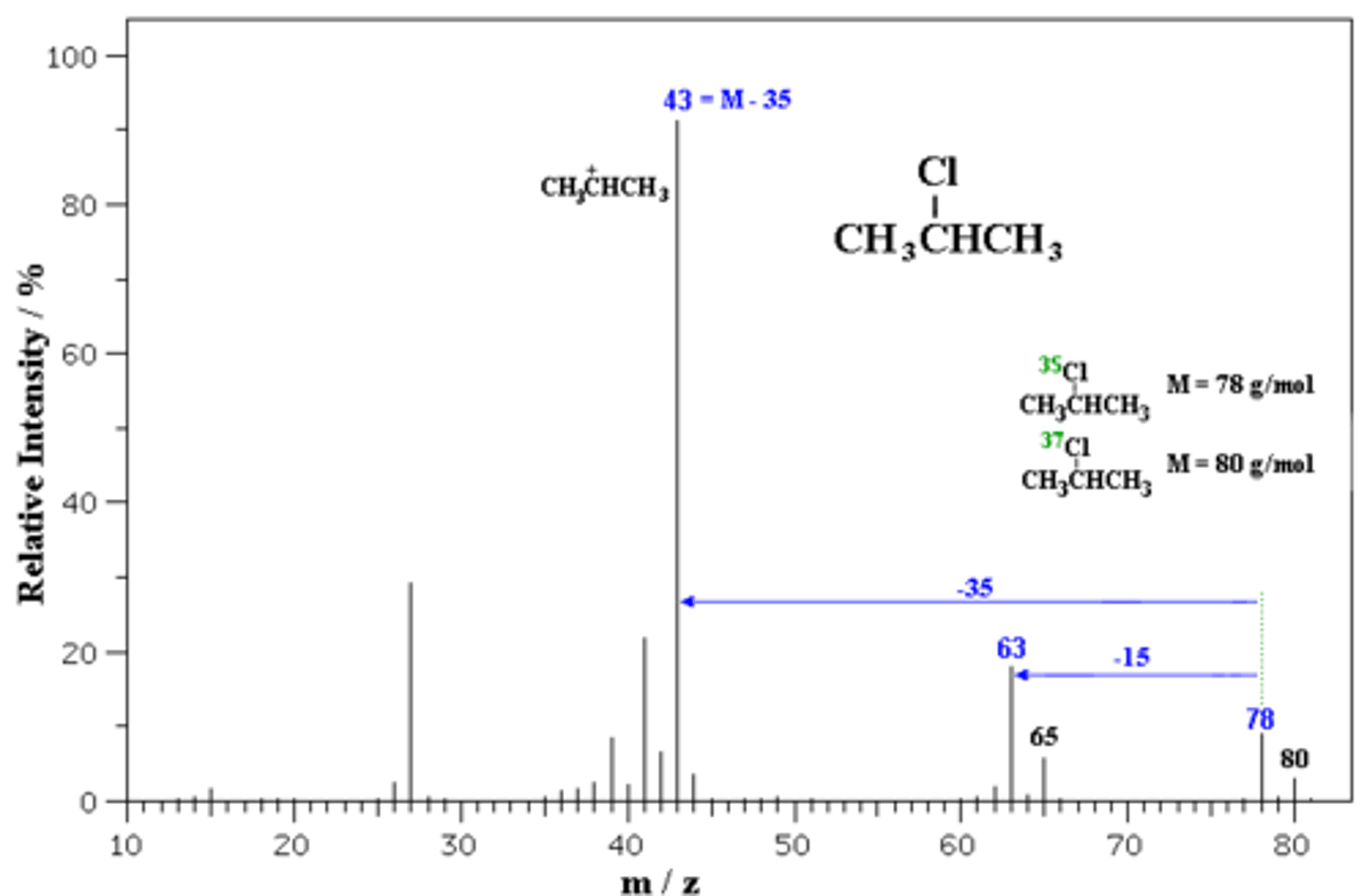
base peak
In a mass spectra, this is the highest intensity peak found. Since a mass spectra is composed of many different fragments, this peak is considered the ion without fragmentation.
ionization
This process is used to give uncharged molecules a charge, since charge is needed for separation in mass spectrometry. There are many ways to induce a charge, such as through dissociation (knocking an electron off or exploding the molecule) as well as forming an adduct.
dissociation
The process of inducing a charge on a uncharged molecule. This process can include knocking an electron off to create a cation, or exploding the molecule to create fragments.
adduct
This is a small, charged ion that is forced onto a uncharged molecule.
M
This designates the weight of a molecular ion.
M⁺
This designates the weight of a molecular ion, but minus one electron. It is nearly the same as the molecular weight.
vacuum
In MS, this is necessary for ensuring that the analyte ions pass through without colliding with anything, including air.
To achieve this, MS instruments use both rough pumps and turbo pumps to pump out air and reach a nearly perfect vacuum.
mean free path
Also known as λ in MS, it is the average distance that a particle travels between successive collisions. Essentially, it is the path of travel from injection to detection.
MS Separation Method #1
This is one of the three methods for separating analytes in MS. This method entails allowing only one m/z to pass through at a time.
MS Separation Method #2
This is one of the three methods for separating analytes in MS. This method entails introducing all analytes at one, and racing them.
MS Separation Method #3
This is one of the three methods for separating analytes in MS. This method entails trapping them in a confined space, and then monitoring their motion.
MS detector
In MS, this monitors the mass per charge of each ion as it exits the mass analyzer.
electron multiplier
This is the most common detector used in MS. This detector is composed of a series of dynodes that each have an increasingly larger electric potential.
As an electron passes through each dynode, the signal is amplified, and the resulting current is measured.
Also: 1 analyte ion makes about 10⁶ electrons.
nominal mass
The integer mass of the most abundant isotope of each element in a molecule.
For example, hydrogen's is rounded to 1.
monoisotopic mass
The exact mass of the most abundant isotope of each element in a molecule.
For example, hydrogen's exact mass is 1.007825.
average mass
The average mass of all the isotopes based on their abundance.
For example, the average mass of the most abundant isotopes for hydrogen is 1.00797, which isn't far off from the monoisotopic mass.
internal standards for MS
In MS, internal standards tend to be deuterated. This leads to similar chromatographic retention, but the m/z will be different in MS.
fragmentation
Also known as mass spectrometry fingerprinting, this is used to help determine the identity of analytes, since many different things have the same molecular mass.
In order to move past this issue, the molecules are fragmented, where each piece is measured.
tandem MS
This is the process of using more than one MS instrument back-to-back. This is meant to perform successive mass analyses, which give a more accurate representation of the analyte.
Essentially, the first round of MS is meant to identify the ion of interest in the sample. We call this ion the precursor ion. From here, the second round is meant to fragment this ion to produce product ions for analysis.
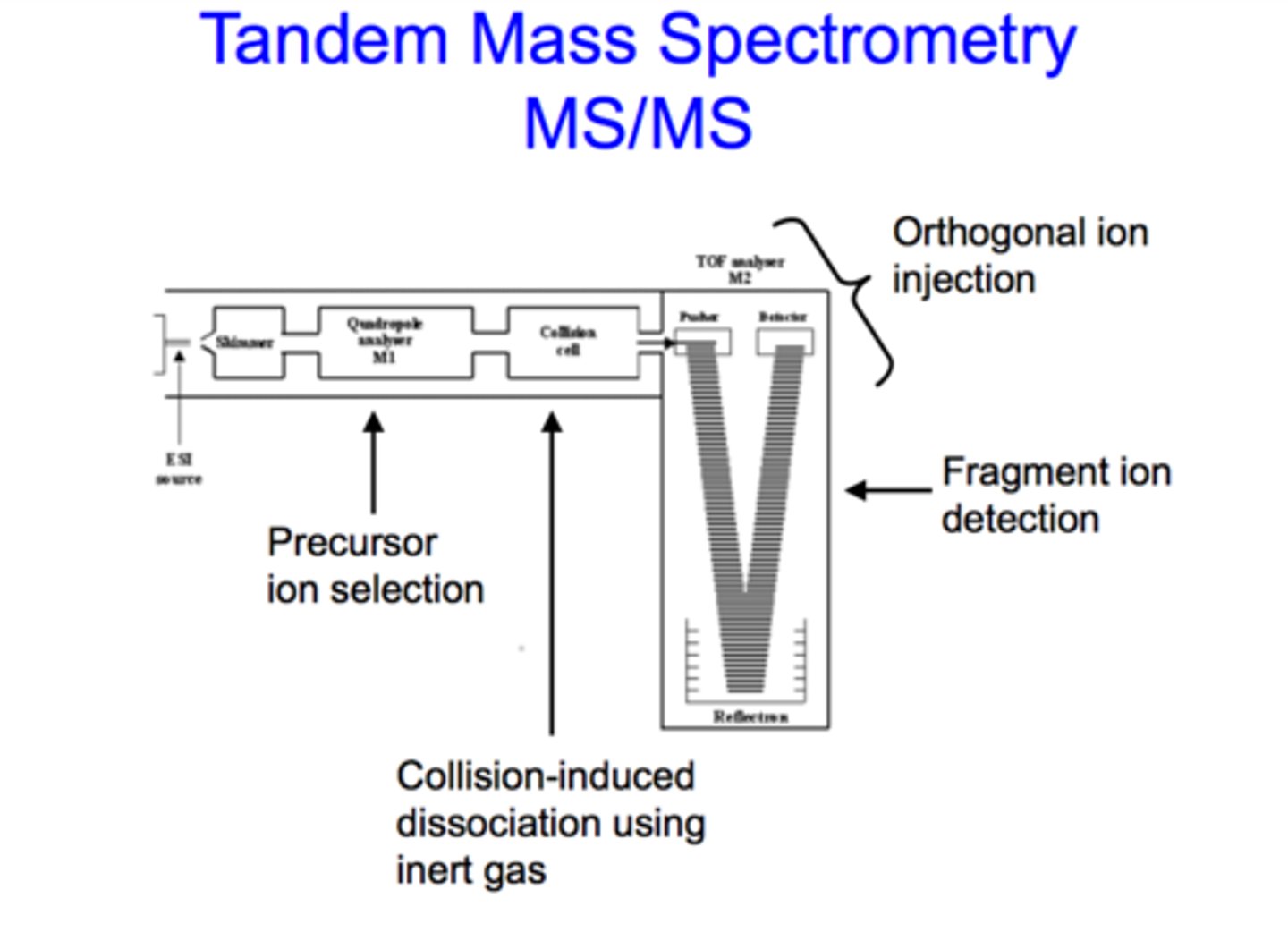
precursor ion
In tandem MS, this is the ion of interest in the original sample. Once selected in the first round of MS, it is then fragmented for analysis in the second round.
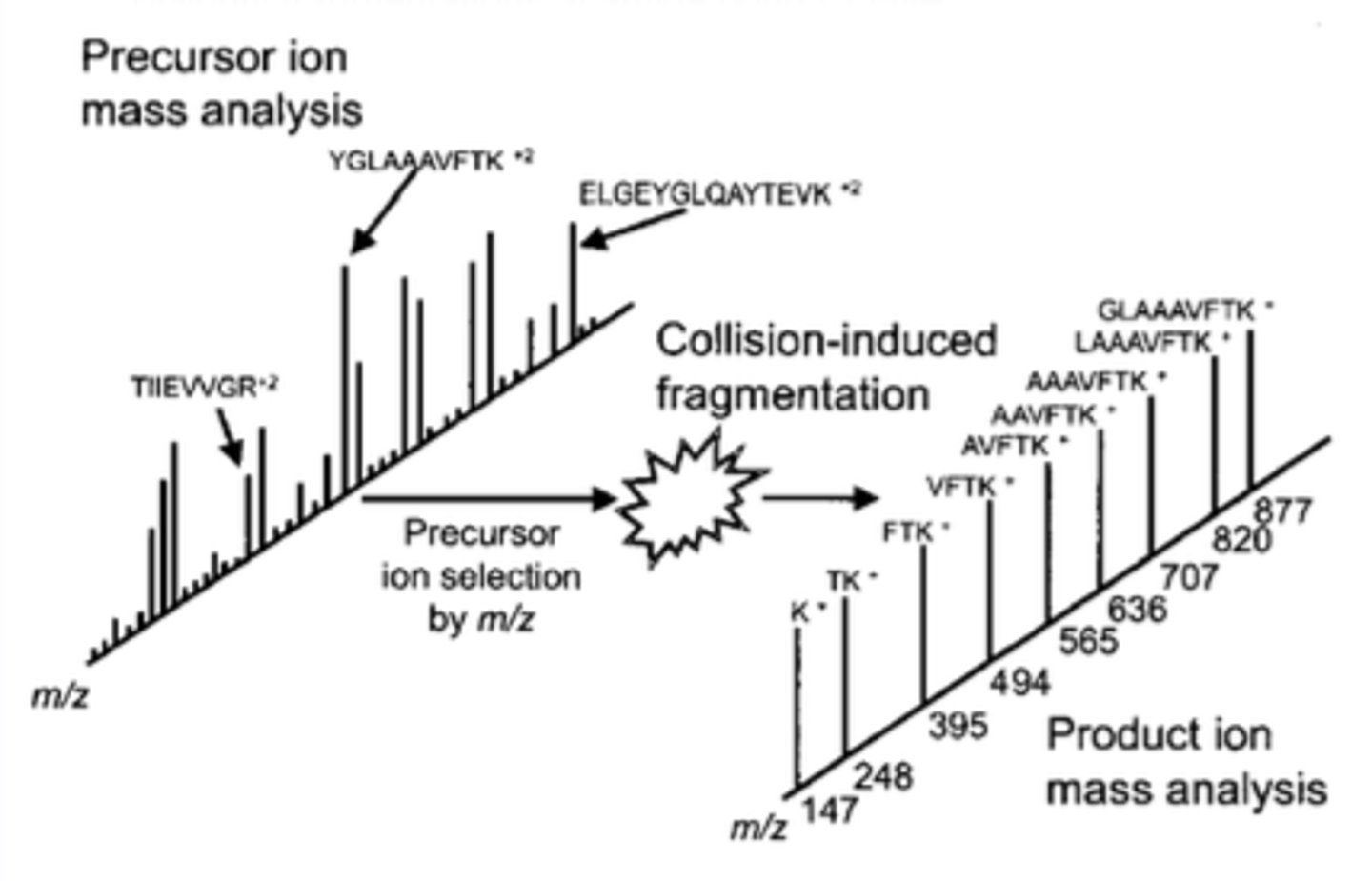
product ions
In tandem MS, these are the fragmented parts of the precursor ion that result from fragmentation in round 2 of MS.
collision-induced dissociation
Also known as CID, this is the most common method of fragmentation. In this method, precursor ions are purposefully fragmented because of their interaction with a gas in the MS.
multiple reaction monitoring
This monitoring is used for selecting only ions that we are interested in. This is done by selecting for specific precursor and product ions, and then monitoring their m/z peaks. This selection can be done with a barrier, which inhibits some precursors or products from moving to the next stage of the MS. An extracted ion chromatogram is the final result.
Because of this, the signal-to-noise ratio is increased, but our LOD is improved, since we are focusing on a few ions of interest instead of the full mass range present in the sample.
total ion chromatogram
This is the chromatogram that show signals from all ions in the mass range.
extracted ion chromatogram
This is the chromatogram that shows signals only from the analytes of interest. These are commonly seen with multiple reaction monitoring.
LC-MS
This is a mass spectrometer coupled with front-end liquid chromatographic separation. In this set-up, the liquid resulting from the LC separation is sprayed onto the mass spectrometer, which is then plotted as the MS signal as a function of retention time.
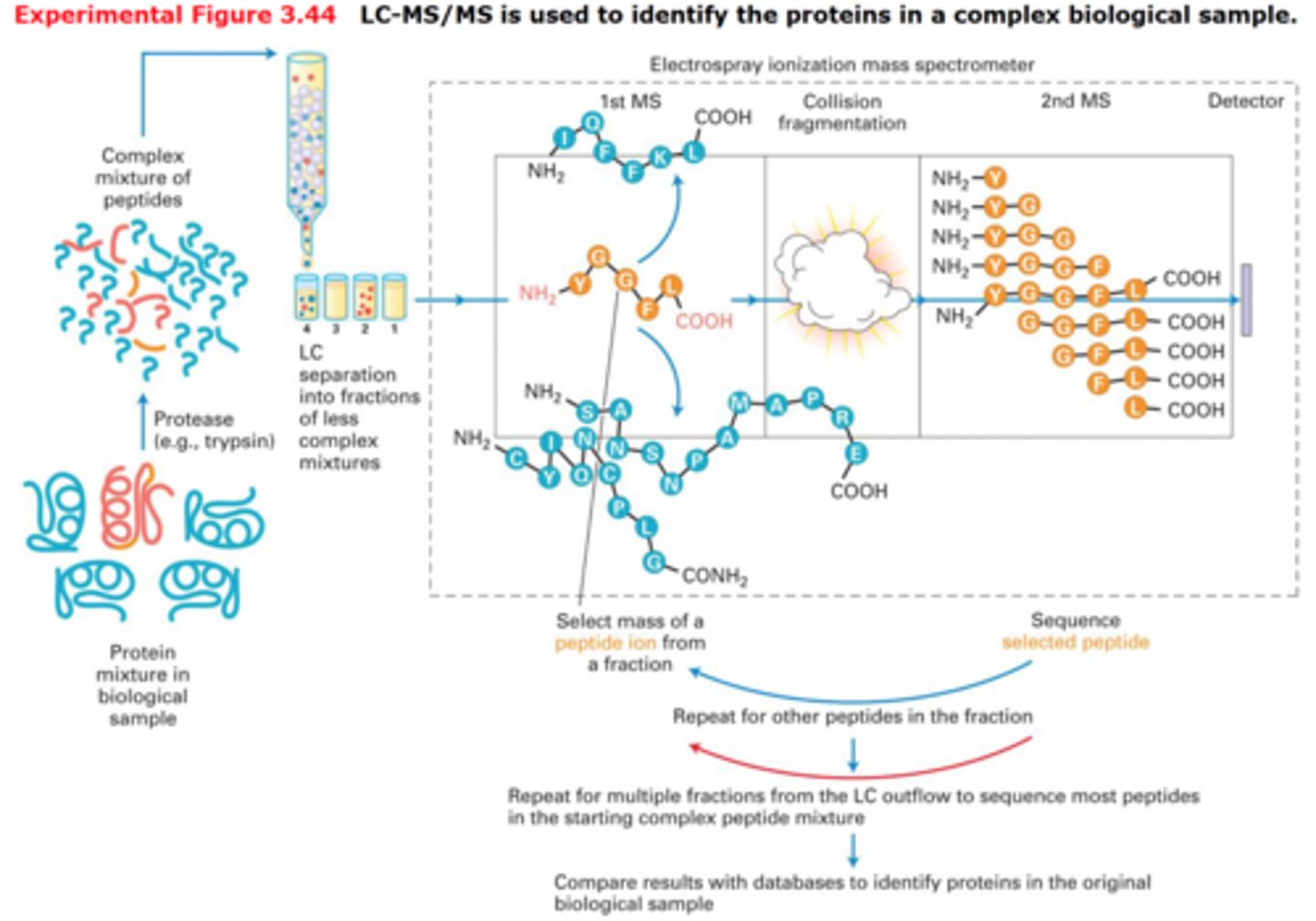
LC-MS/MS
This is common for most routine analyses in industry or medicine, since this tandem instrument uses multiple reaction monitoring. For instance, we would use this when we are looking for a specific biomarker in blood, or are conducting quality control testing, product development, or drug research.
All-in-all, we already know what we want to look for, so this instrument does the job we need.
benefits of mass spectrometry
Among the other instruments we've seen for analytical purposes, the benefits of MS are:
1. It gives the identify of an analyte (no worrying about retention times being the sole evidence).
2. It has a high resolution.
3. It is not guaranteed, but it can analyze nearly anything in any phase of matter.
hard ionization
This is one of the many techniques used to create charged ions for MS analysis. This technique puts high energy on a molecule to create major fragmentation. This is good for determining structure.
soft ionization
This is one of the many techniques used to create charged ions for MS analysis. This technique puts low energy onto a molecule to create minor fragmentation. This is good for determining molecular weight.
electron impact
Also known as EI, this is one of the applications of hard ionization. This application involves shooting an electron beam at an analyte. The electron beam will knock-off an electron, which makes the analyte cationic. This helps determine the structure of the analyte.
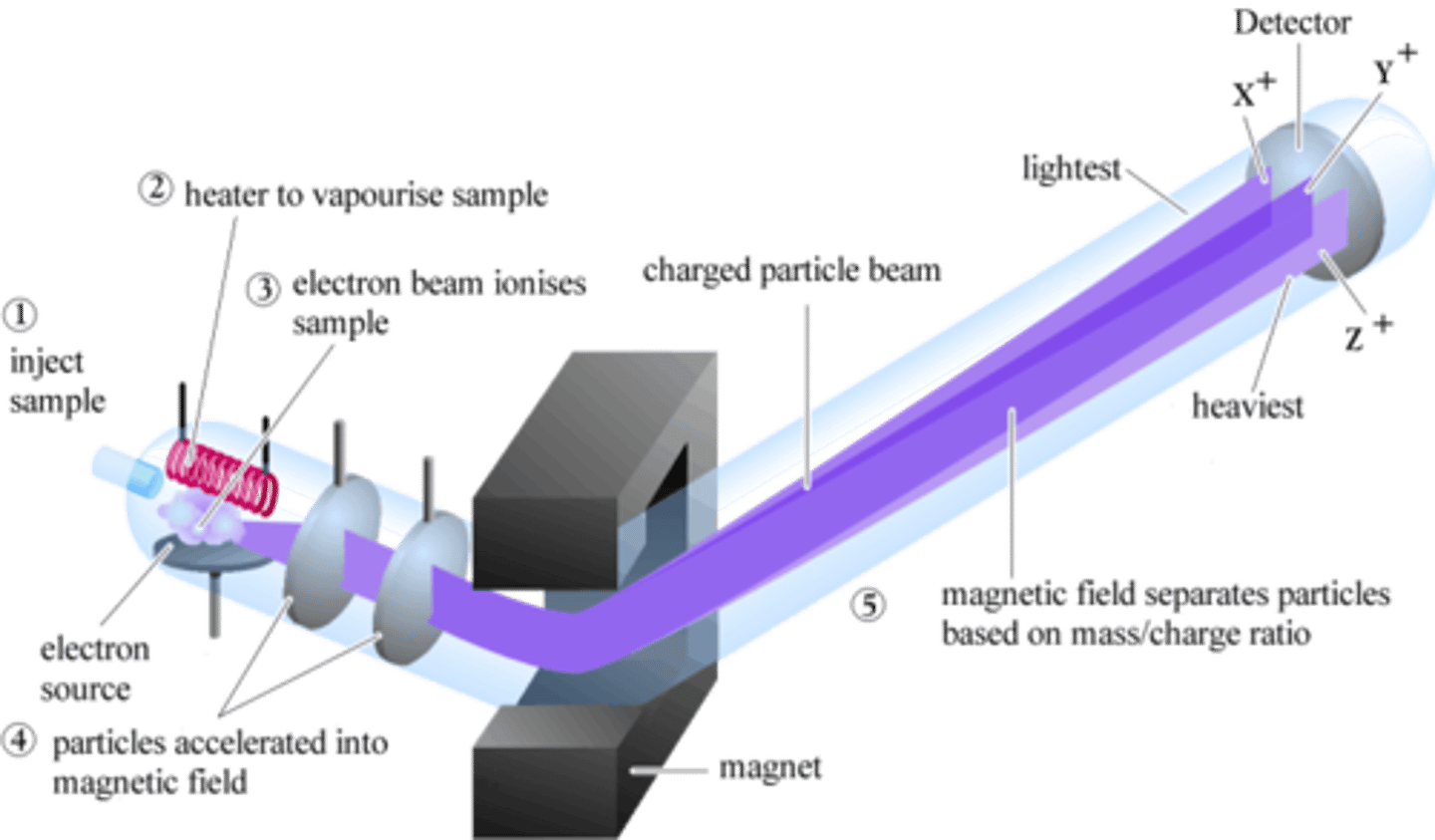
EI: Step 1
The first step of EI is the producing electrons from an incandescent filament.
EI: Step 2
The second step of EI is application of a voltage between the filament and top of the chamber. This accelerates the electrons towards the sample.
EI: Step 3
The third step of EI is introducing the sample perpendicularly, so it will be hit by the electrons.
EI: Step 4
The fourth step of EI is the newly cationic analyte molecules being repelled by the electric field, since they are now charged. This directs them towards the MS for analysis.
limitations of EI
The limitations of EI include:
1. Only gas phase analytes can be used
2. Only small molecules
3. There is high energy dissociation, so some ions may be absent
4. Needs high heat and vacuum
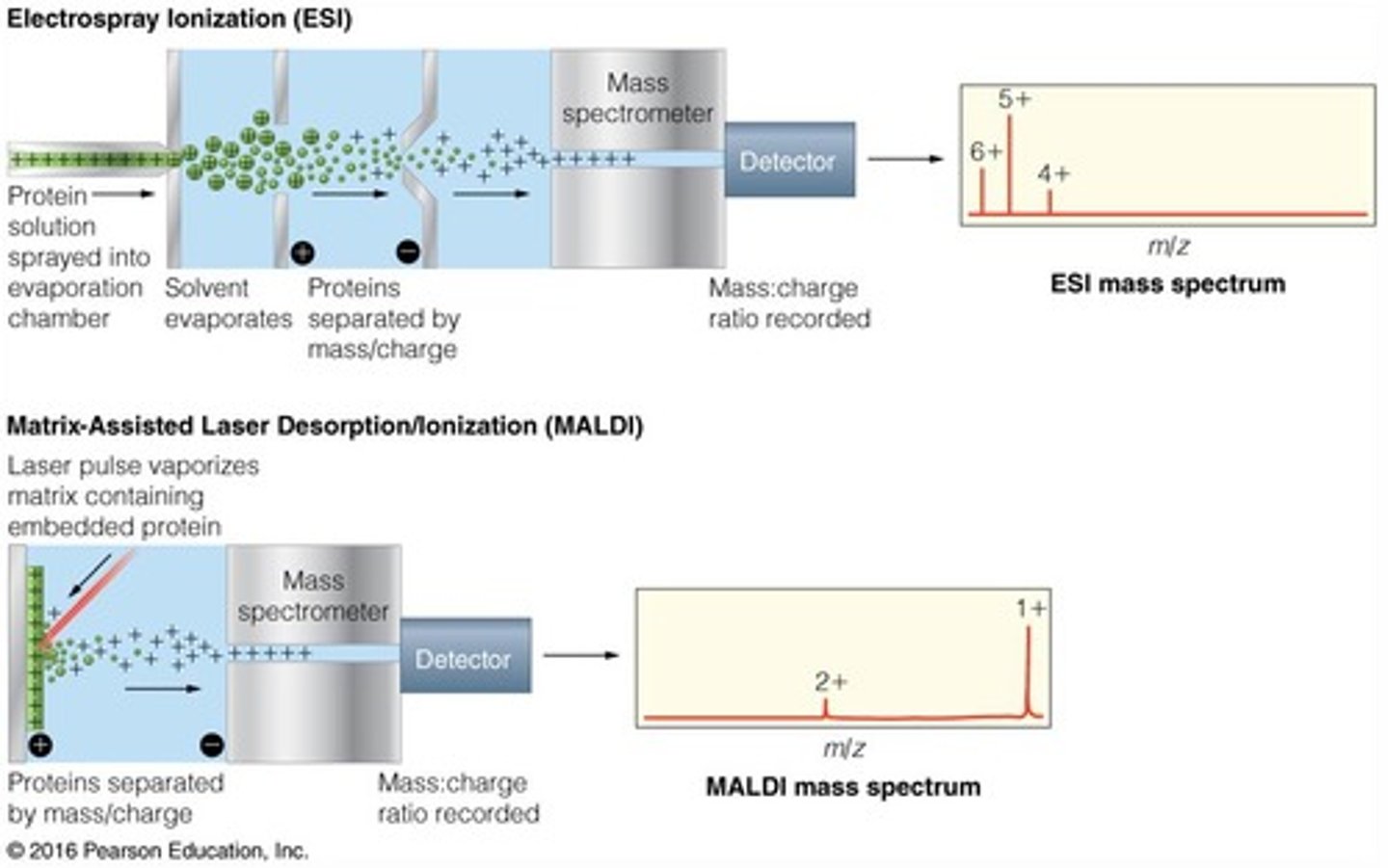
matrix-assisted laser desorption ionization
Also known as MALDI, this is one of the applications of soft ionization. This application involves analyzing large biomolecules (larger than 200 kDA) suspended in a matrix with MS.
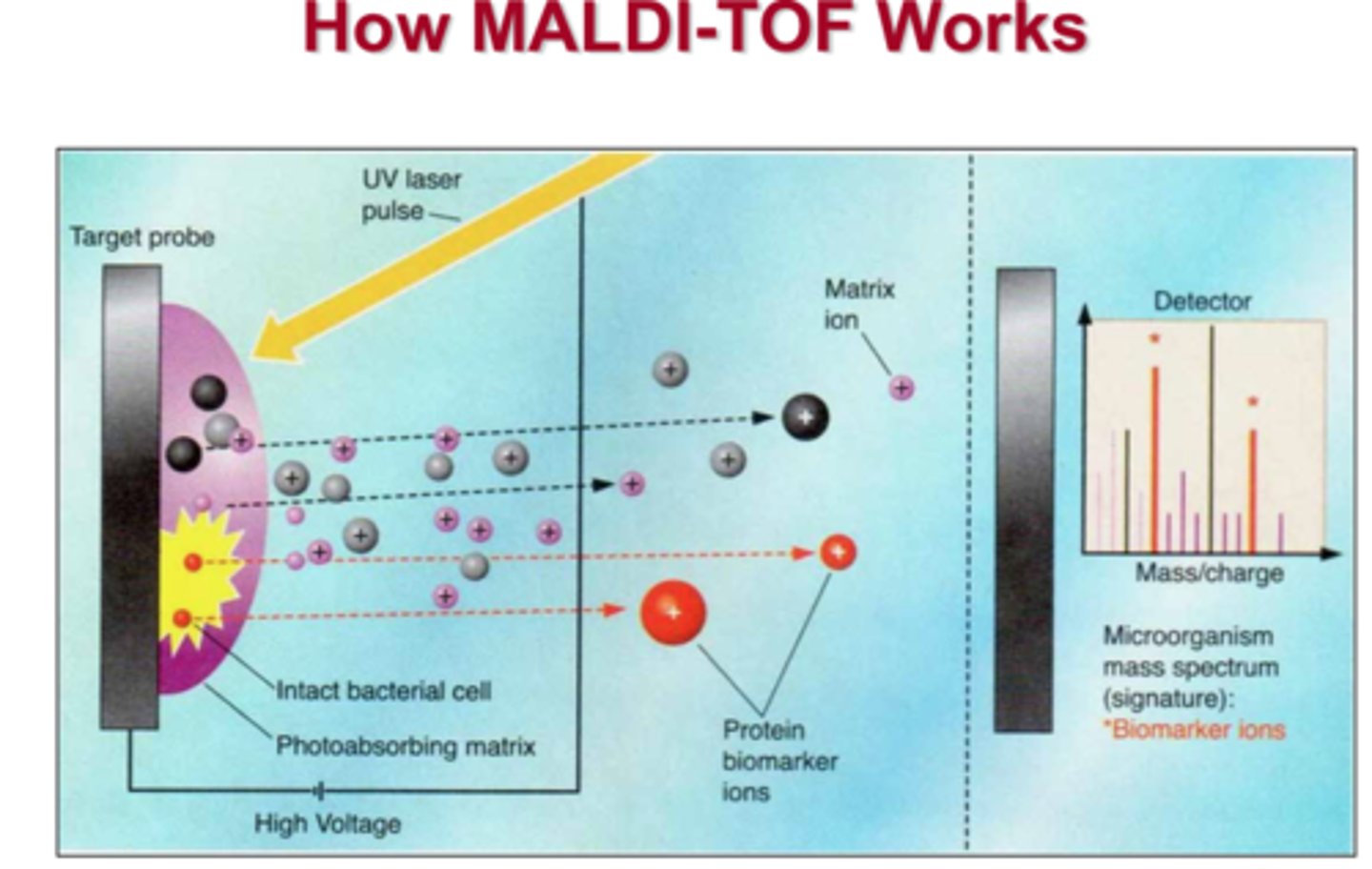
MALDI: Step 1
In the first step of MALDI, a sample is mixed with matrix material. This is done to help absorb large amounts of energy that come from the laser, which is then used to help desorb and ionize analyte molecules into the gas phase. Ultimately, this forms an adduct.
MALDI: Step 2
In the second step of MALDI, the sample coated with matrix is shot with the laser. The matrix absorbs the energy, which desorbs and ionizes the analyte into the gas phase. Here, an adduct is formed.
MALDI: Step 3
In the third step of MALDI, the newly-formed adduct is then directed towards the MS for analysis.
MALDI considerations
The considerations to take into mind with MALDI are:
1. There is a lot of noise at low mass, because the amount of analyte in the matrix is much lower than the matrix itself.
2. It is hard to use front-end separation, since samples have to be coated with matrix.
3. The MS used needs a large mass range.
4. The matrix can be nonuniform, which can affect signals.
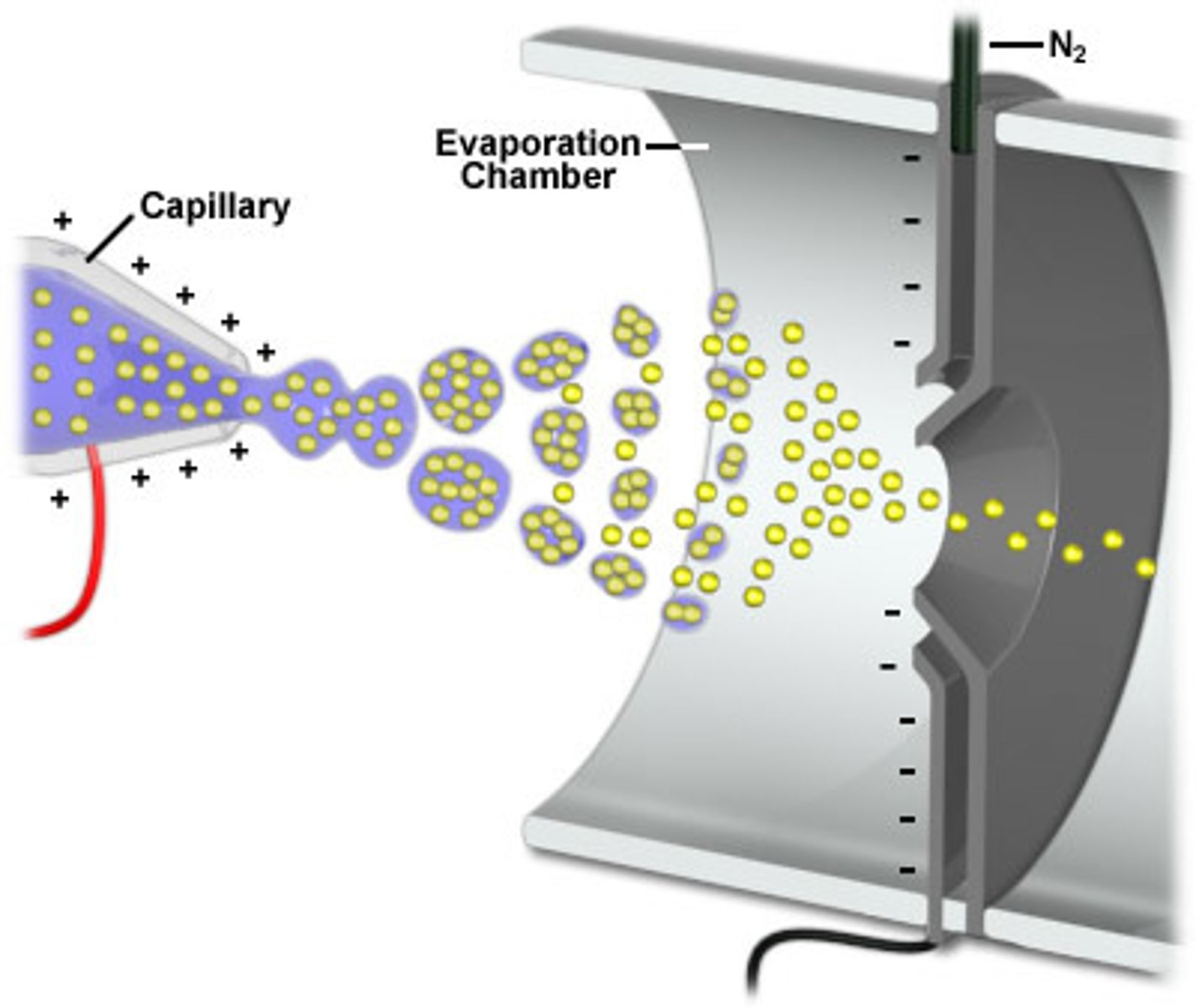
electrospray ionization
Also known as ESI, this is one of the applications of soft ionization. This application is used for inducing charge on liquid samples and analyzing large molecules.
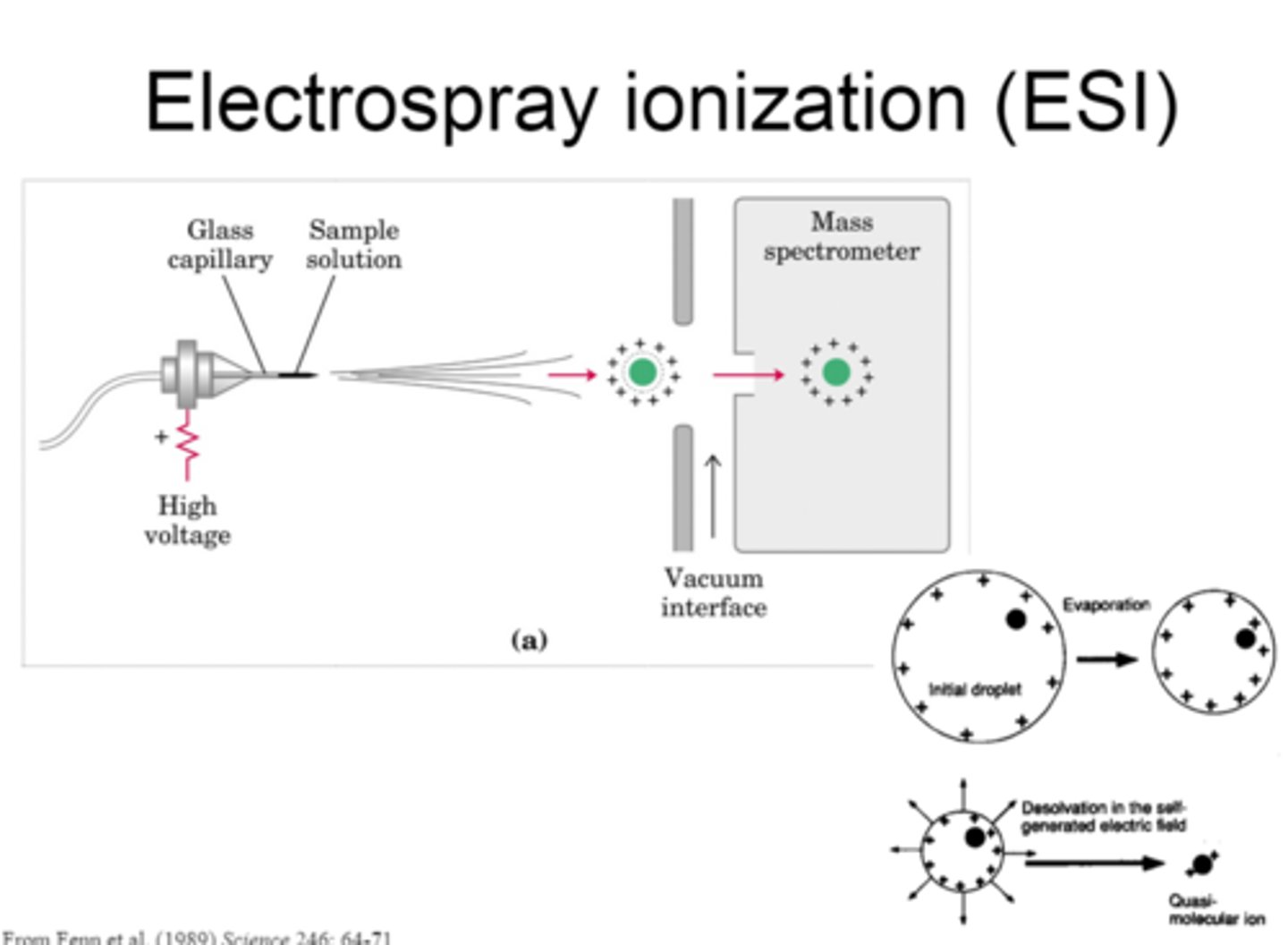
ESI: Step 1
In the first step of ESI, the sample solution flows through a nebulizer capillary, which creates a fine spray.
ESI: Step 2
In the second step of ESI, a potential is applied to the capillary, which induces a charge on the spray.
ESI: Step 3
In the third step of ESI, desolvation is performed to remove the solvent and form an adduct.
ESI: Step 4
In the fourth step of ESI, the electric field is used to draw the adducts into the MS.
ESI considerations
Considerations for ESI are the following:
1. It cannot be used for gas-phase or solid-phase analytes.
2. Deconvolution can be complex because there can be many charged states for a single analyte. However, front-end separation can help this.
3. Not much structural information is gained.
4. Salts produce problems by suppressing ionization and forming multiple adducts.
desorption electrospray ionization
Also known as DESI, this is very similar to ESI, but it can be used in solid-phase samples as well as liquid-phase samples. No solution flow is required, the samples are kept in ambient air, and both small and large molecules can be analyzed.
DESI is performed by electrospraying charged solvents onto a sample/surface. Then, the desorbed ions are transferred to the MS.
Essentially, this can be used for on-site analyses.
inductively coupled plasma
Also known as ICP, this is one of the applications of hard ionization. This application involves passing an analyte through plasma, which contains ionized gas and electrons. Ultimately, this produces very hard ionization, leading to structural analysis of the analyte.
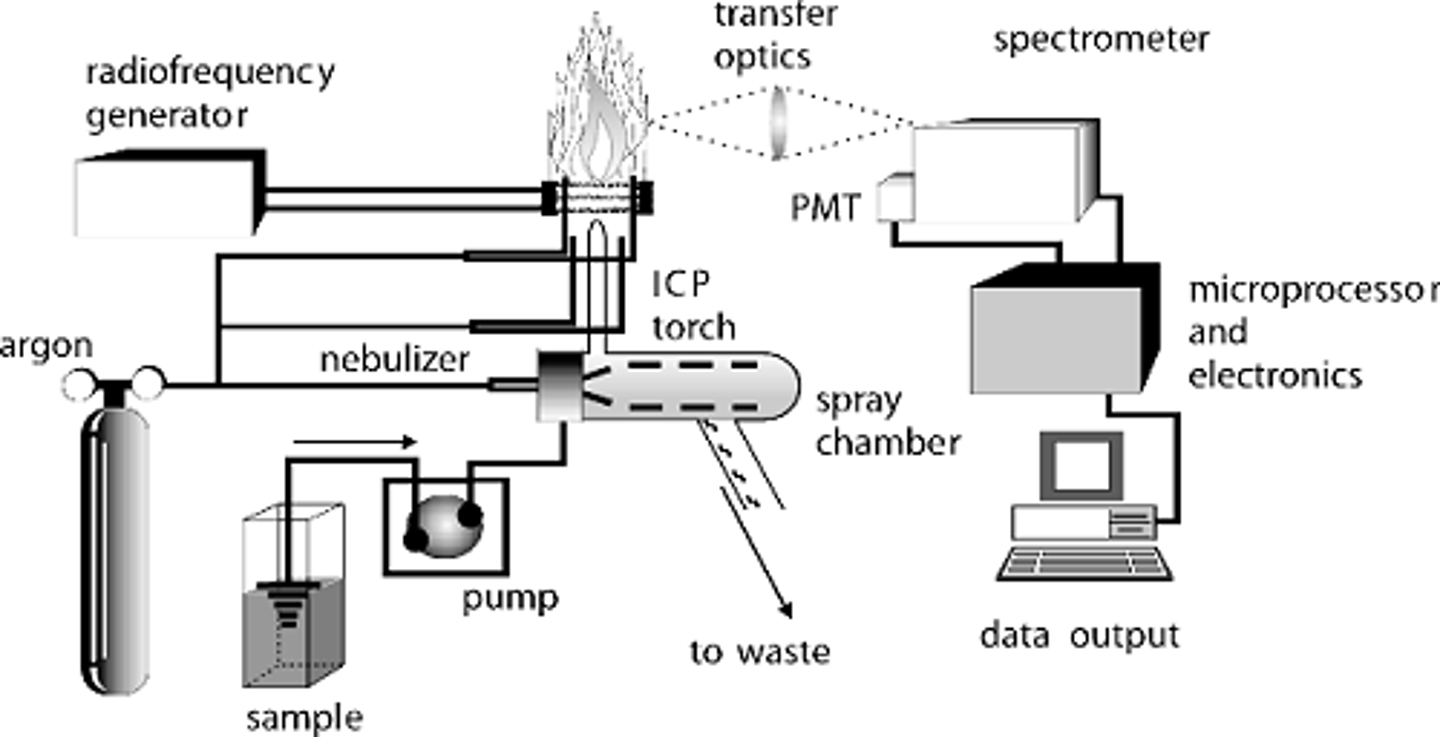
ICP considerations
The considerations of ICP are the following:
1. A sustained plasma (around 8000 K) has to be sustained.
2. Liquid-phase and solid-phase samples can be run.
3. It can be hard to have a high flow rate with the plasma torch.
mass analyzer
This is the instrument which actually does the mass spectrometry by physically separating ions based on their m/z ratio. There are many forms that you different things like momentum, kinetic energy, or velocity to help separate.
mass range
The maximum m/z an instrument can analyze.
For multiply-charged ions, a low mass range can be overcome.
resolution
In MS, this is the ability to separate two neighboring mass ion peaks.
mass accuracy
The error in the measure m/z. This is relative to the theoretical mass of the analyte.
speed
The number of spectra per unit time.
This should increase as the speed of the front-end separation increases.
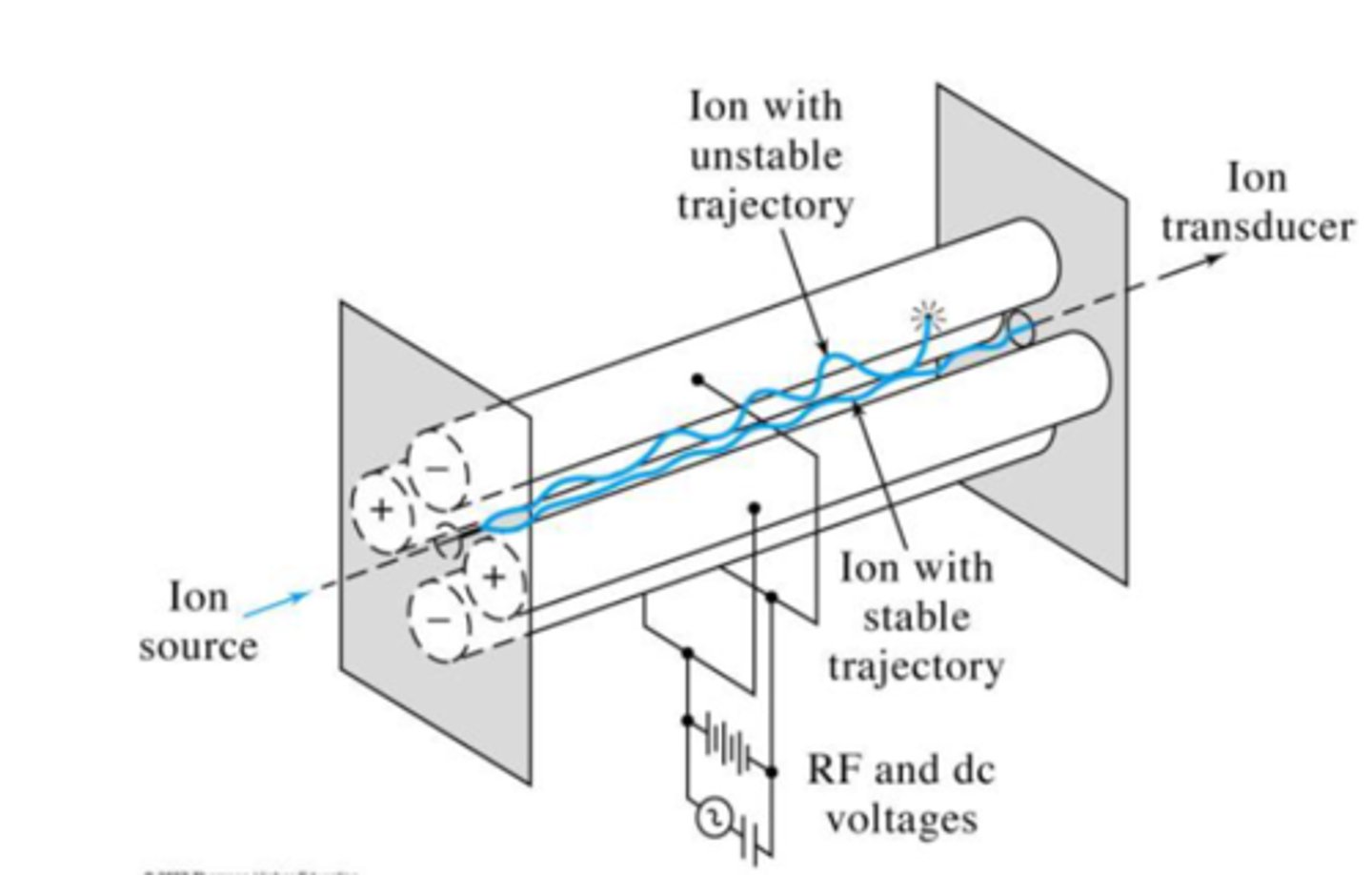
quadrupole
This is the most widely used mass analyzer. It is composed of four precisely machined, parallel, conductive rods. A DC current and RF field is applied, which helps separate particles by affecting their path stability from injection to detection.
Ions that are affected by the DC and RF field collide into the poles and are not detected (non-resonant ions), while those which are not as affected continue to the detector (resonant ions). By manipulating the voltages of both the DC and RF field, we can scan for different ions.
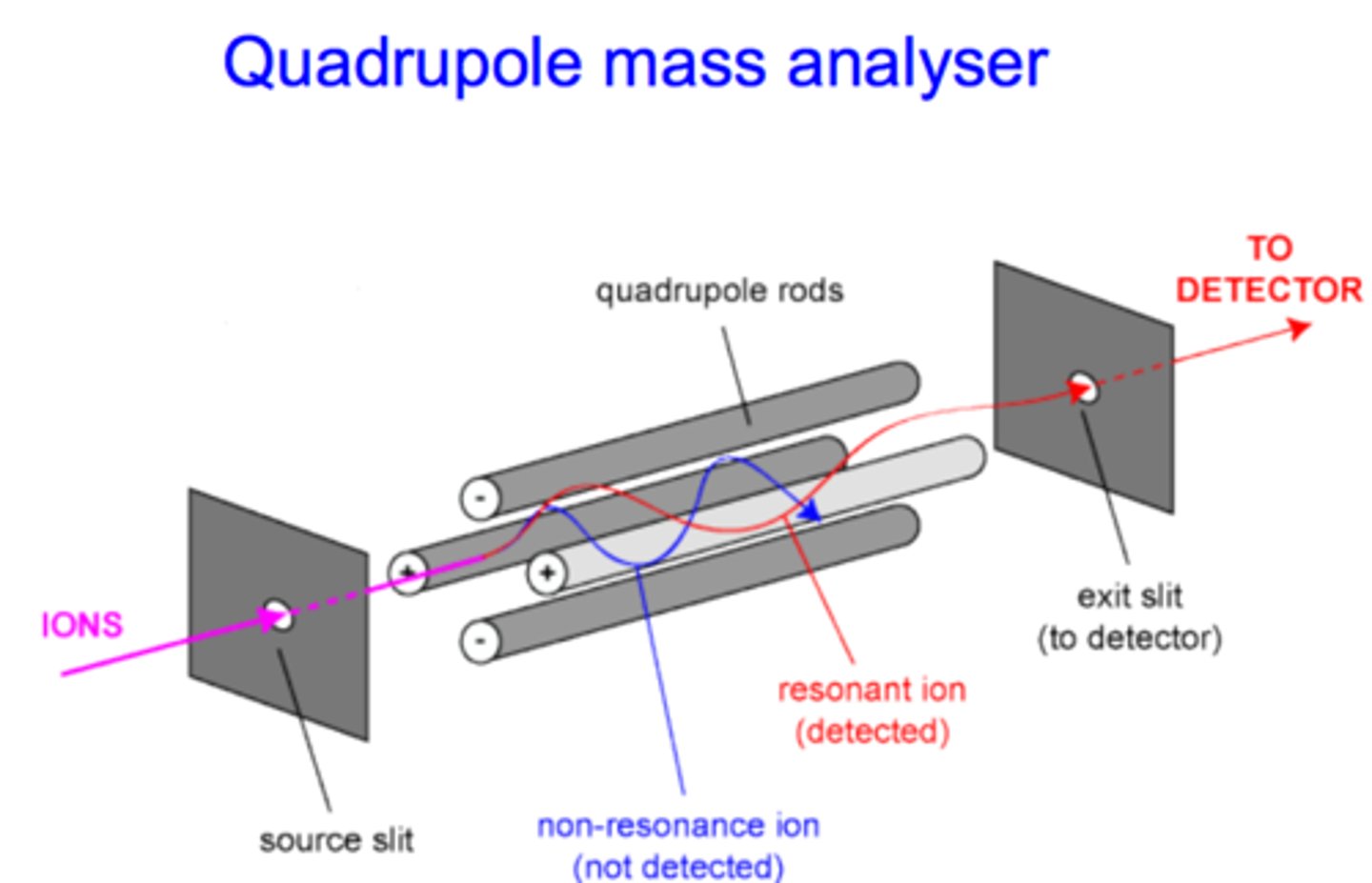
resonant ions
Ions with stable trajectories that can make it through the quadrupole and onto the detector. Their stable trajectories mean the DC and RF field do not have a large effect on them.
non-resonant ions
Ions with unstable trajectories that cannot make it through the quadrupole and onto the detector. Their unstable trajectories mean the DC and RF field do have a large effect on them.
DC
This stands for direct current. This type of current is used in a quadrupole for separating ions.
RF
This stands for radio frequency. It is composed of an alternating current and sine wave, and is used in a quadrupole for separating ions.
+V
In a quadrupole, this voltage is used in rod pairs as a high-pass filter.
-V
In a quadrupole, this voltage is used in rod pairs as a low-pass filter.
quadrupole considerations
The considerations for using the quadrupole are the following:
1. It is compact and relatively inexpensive (when compared to other instruments).
2. It has a limited mass range.
3. It has limited resolution.
triple quadrupole
Also known as QQQ or the triple quad, this is three quadrupoles put together. The first and third quadrupoles are where the actual separations occur, while the second one is meant for fragmentation.
All-in-all, the triple quad is the best MS technique for quantitative analysis, and it has great sensitivity when doing selected- or multiple-reaction monitoring.
Q1
In a triple quadrupole, this is the first quadrupole. This one is used for mass selection and has a normal mass filter.
Q2
In a triple quadrupole, this is the second quadrupole. Here, CID and a RF field is only used. Essentially, this quadrupole is just used for fragmentation between Q1 and Q3.
Q3
In a triple quadrupole, this is the third quadrupole. Here, a MS scan is completed with a normal mass filter for the fragmented ions from Q2.
reaction monitoring
The act of selecting a specific ion, fragmenting it, and detecting one of the fragments. There are two kinds of this: multiple reaction monitoring and selected reaction monitoring.
ion monitoring
The act of selecting and detecting only your ion of interest. This does not include any reaction, and therefore, there is no fragmentation.
time-of-flight
Also known as TOF, this is the most widely used analyzer for analysis of biomolecules.
In this methodology, ions are separated by differences in velocity, which is related to their masses. The smaller the mass, the faster the velocity, which means faster arrival times.
TOF considerations
Here are the following considerations for TOF:
1. Needs precise ion introduction (have to enter at same time).
2. Need to be coupled with pulsed ionization source (like MALDI)
3. Nearly unlimited mass range
4. High mass resolution
5. NOT a scanning instrument
quadrupole ion trap
Also known as QIT, this is an application of using a quadrupole to separate ions. In this application, ion separation occurs by manipulating ions in time rather than space by trapping them in an electric field.
Once trapped in this oscillating electric field, each ion is detected by ejecting them sequentially.
QIT considerations
The following are considerations for QIT:
1. It is simple, robust, and inexpensive.
2. It needs a pretty low vacuum.
3. Low accuracy and mass range.
4. Limited ion storage capacity.
5. Not ideal for quantitation.
spectroscopy
The interaction of nuclei, atoms, ions, or molecules with electromagnetic radiation. This interaction varies from emission to absorption to scattering.
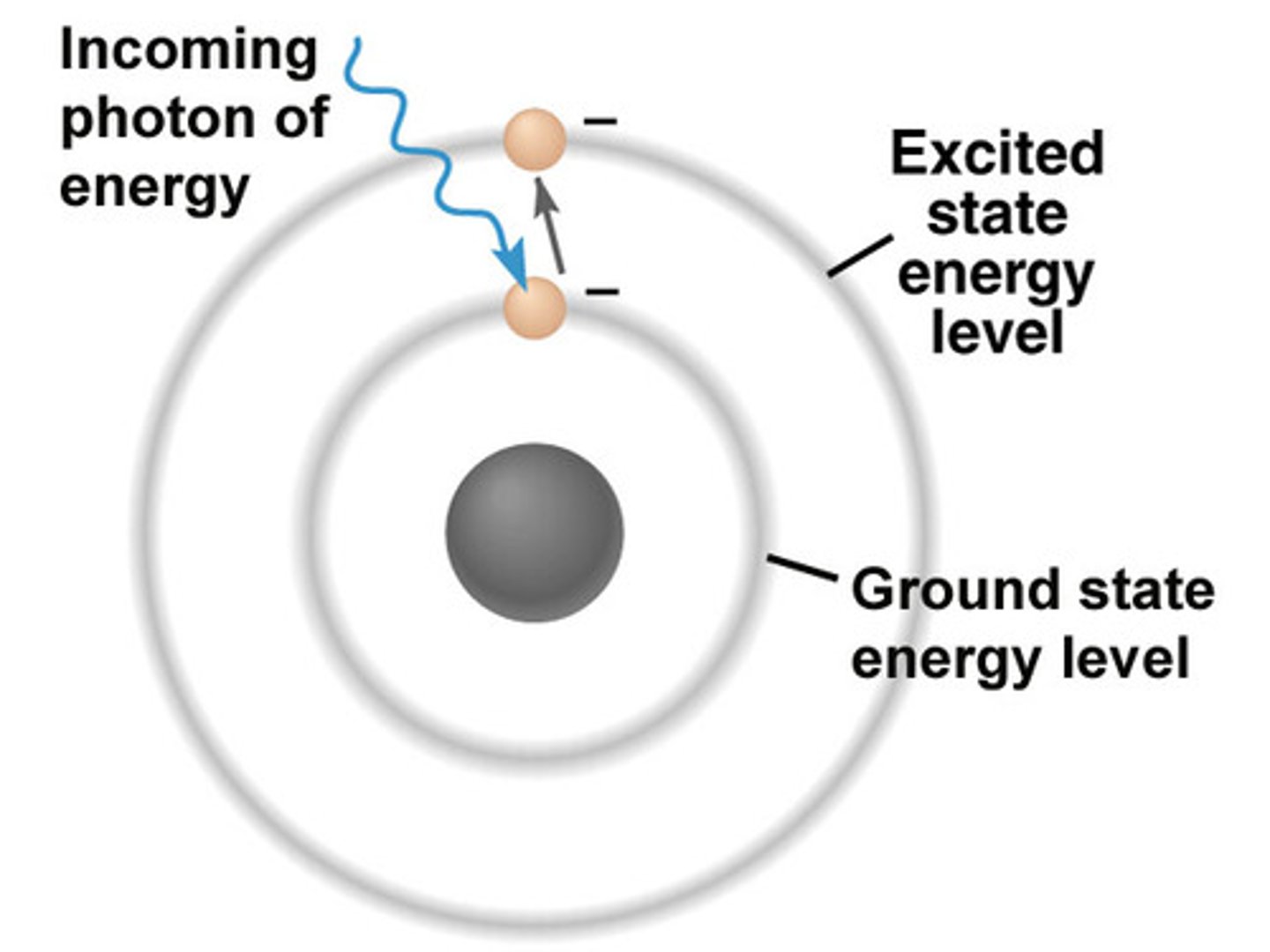
absorption
The excitation of species by absorbing EM radiation. A molecule may absorb EM radiation if the energy levels of both are matched. If they are, then the molecule goes from the ground state to the excited state.
ground state
This is the state a molecule's electrons are originally in before absorbing energy.
excited state
This is the state a molecule's electrons are in after absorbing energy.
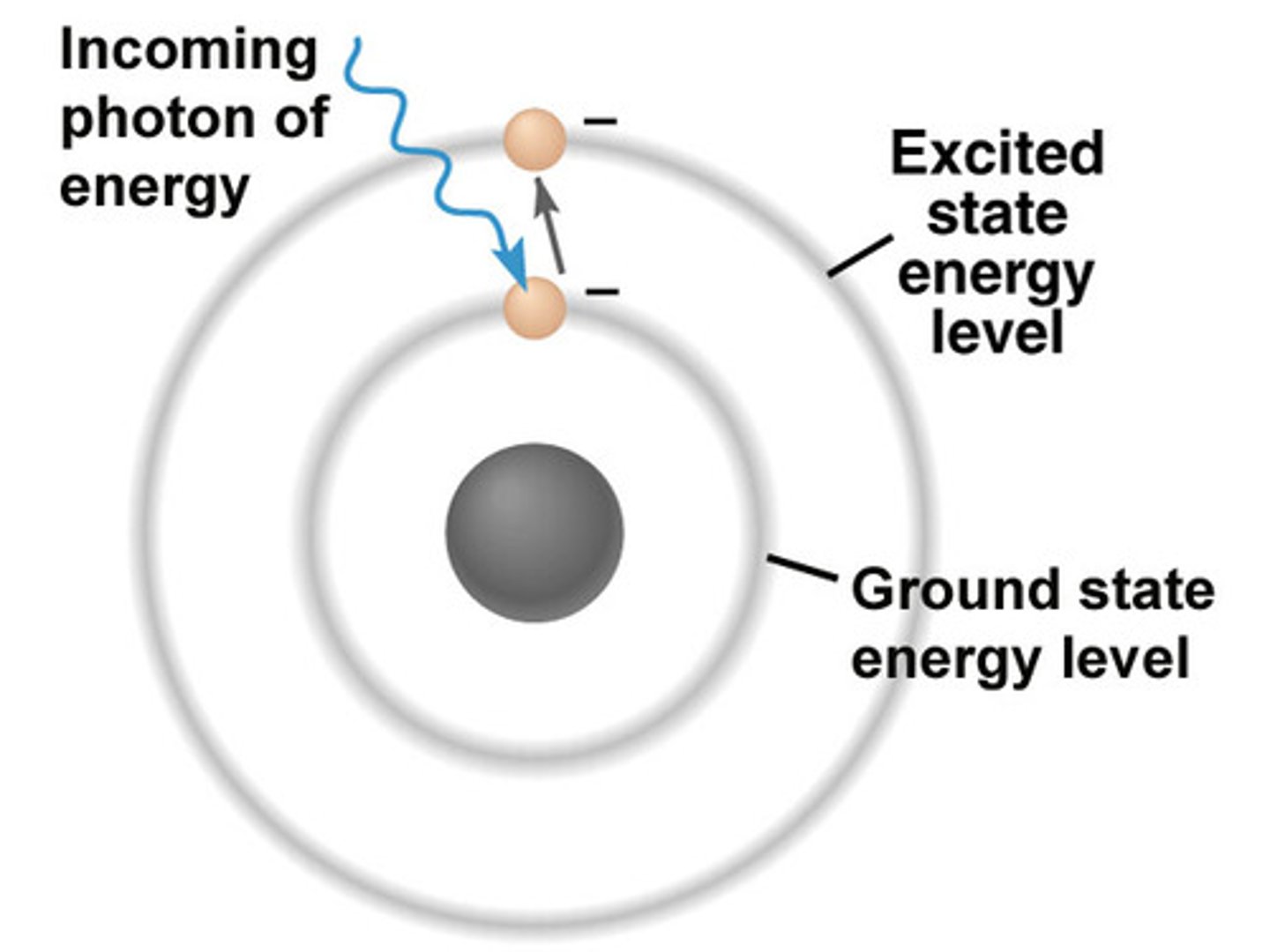
emission
The release of EM radiation from excited atoms. Applications include luminescence, fluorescence, and phosphorescence.
scattering
Low frequency energy modes.
amplitude
This is the intensity of a wave. It is measured from the top of a peak to the base.
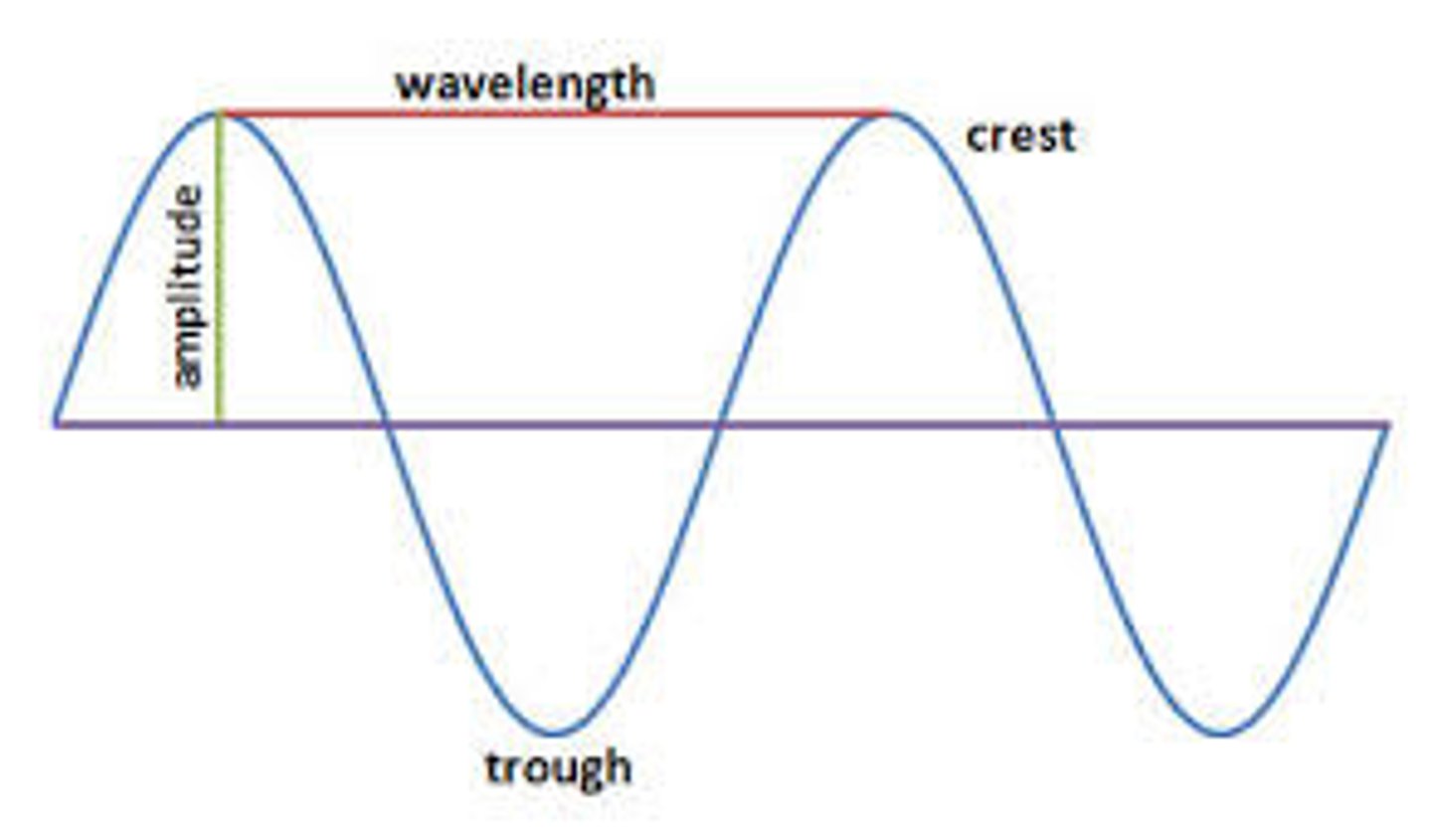
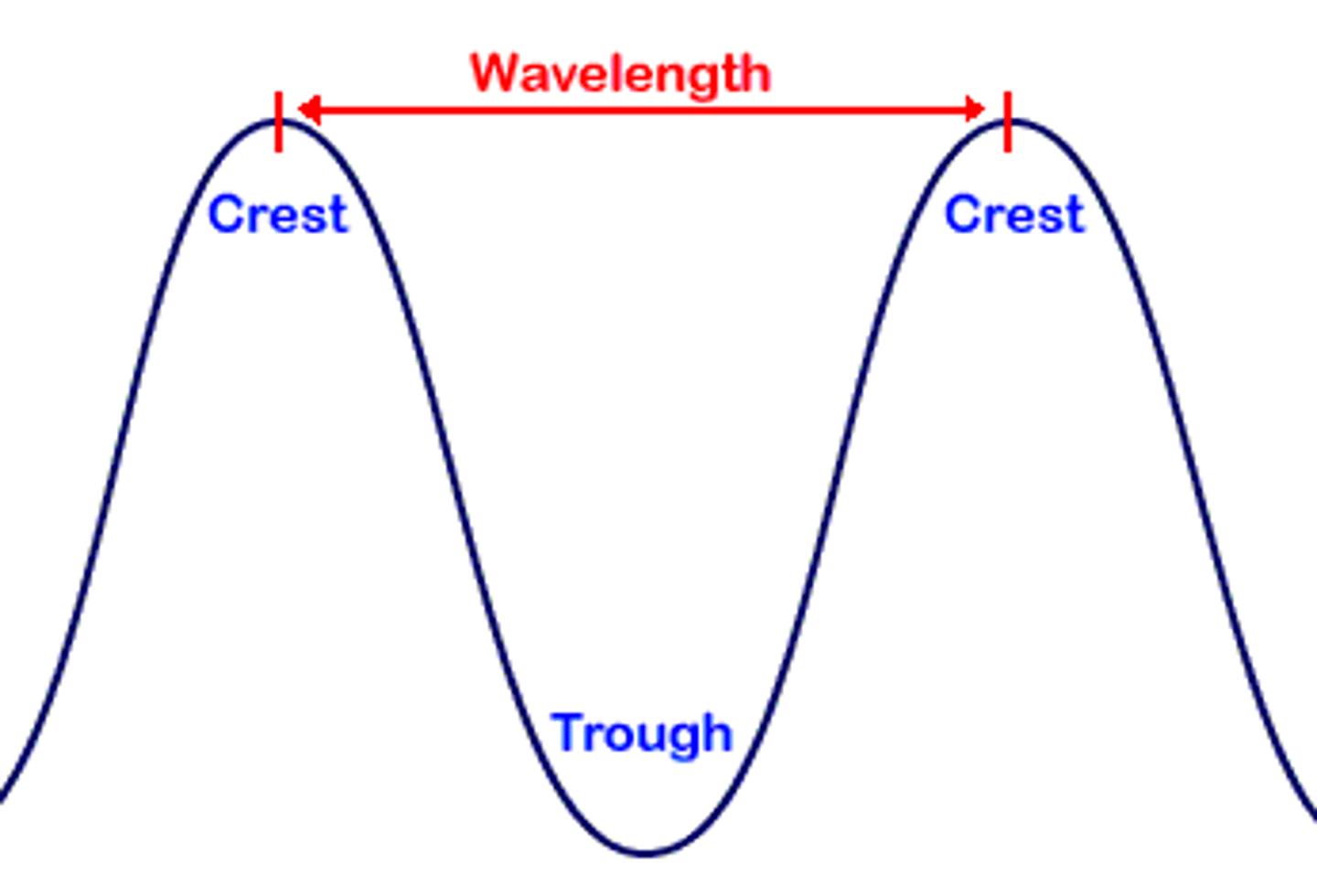
wavelength
Also known as λ, this is the distance between the peaks in a wave. Usually, it is presented in nanometers.
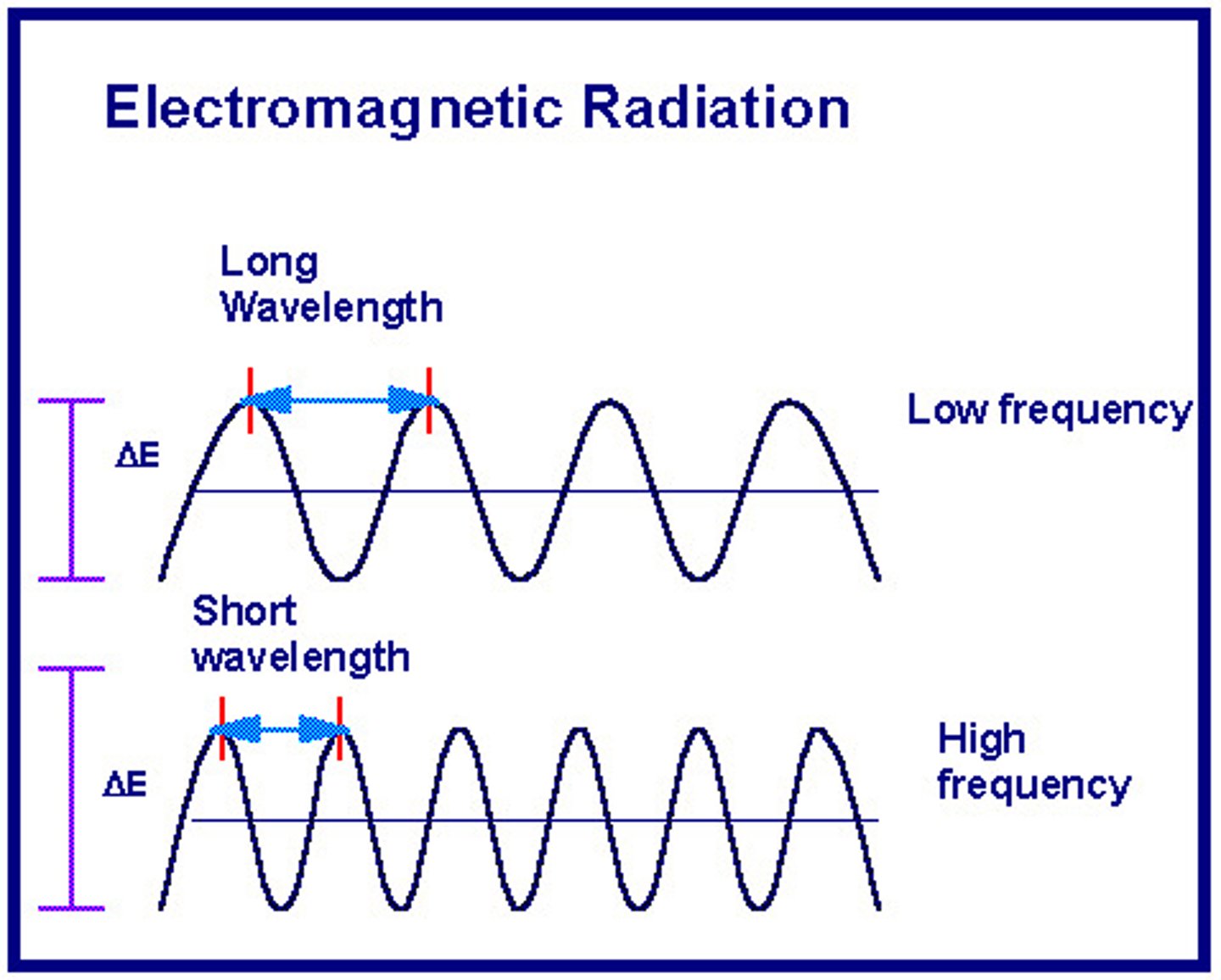
frequency
Also known as v, this is the number of wave oscillations per time. Usually, it is presented in Hertz.
Hertz
Also known as Hz, these are equivalent to s⁻¹ seconds. It is the unit for frequency.
velocity
Also known as c, this is the speed of light. Usually, it is measured in meters per second, and is kept constant.
c = 2.9998 x 10⁸ m/s
Planck's constant
Also known as h, this constant is used for solving for either energy, wavelength, or frequency.
h = 6.626 x 10⁻³⁴ J x s
equation for energy
E = hv = hc/λ
microwave energy
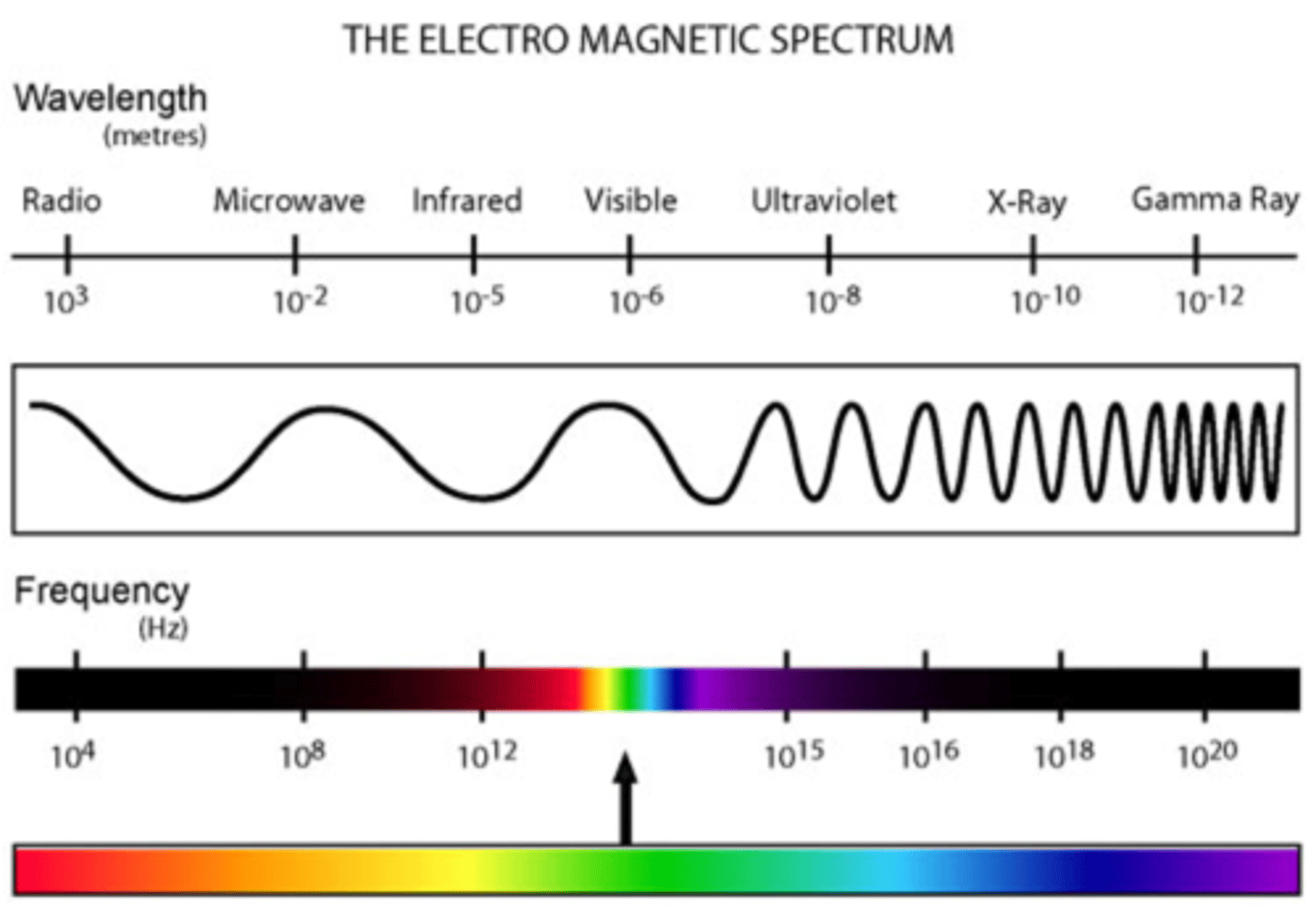
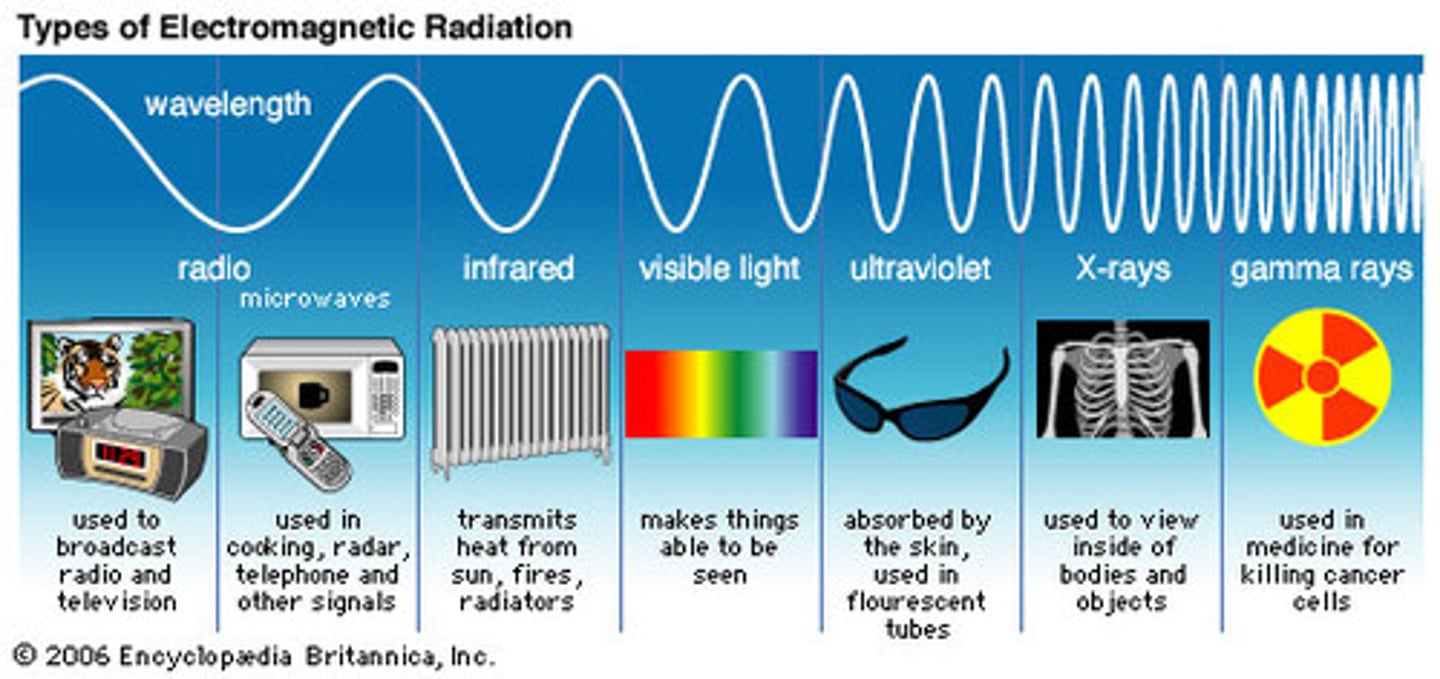
This form of EM energy has wavelengths of around 1 mm to 1 meter and frequencies of 300 GHz to 300 MHz. This EM energy rotates molecules, which is useful for things like heating food. Also, because of the long wavelengths, it can be used in long-range communication.
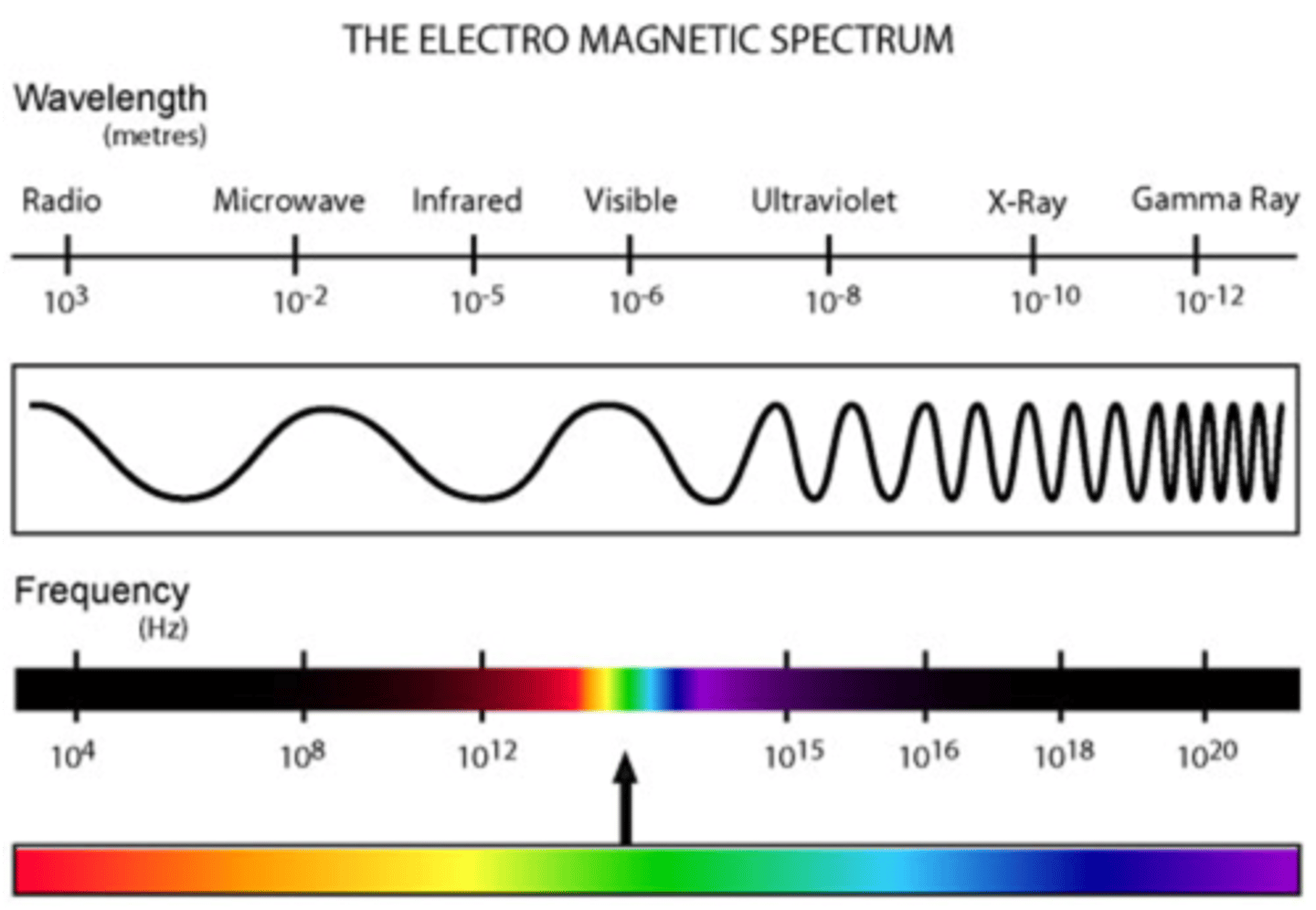
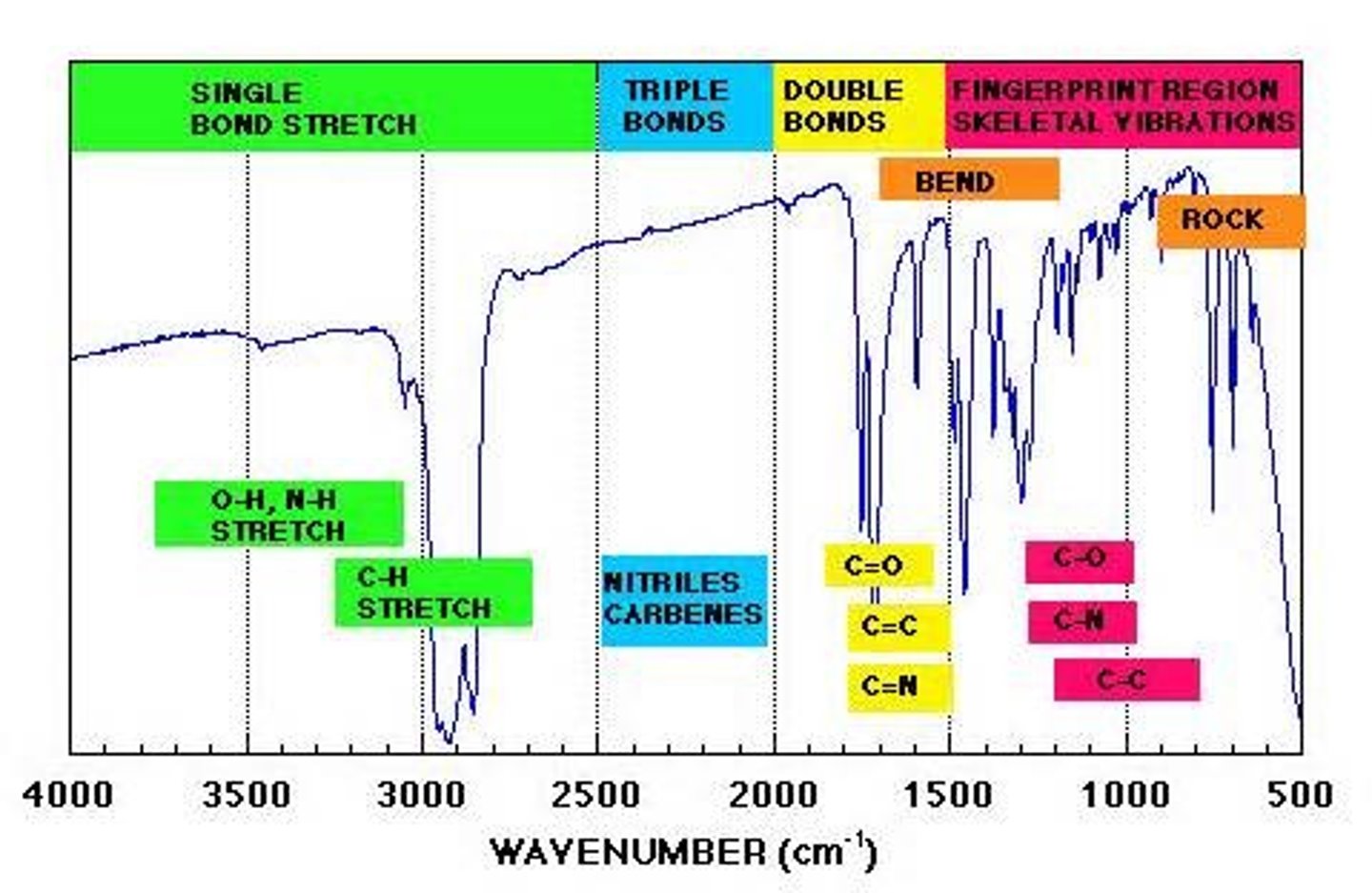
infrared energy
This form of EM energy has wavelengths of around 750 nm to 1 mm and frequencies of 400 THz to 300 GHz. This EM energy vibrates molecules, which includes bending, stretching, wagging, and twisting* of the bonds.
We often see this applied in determining the identity of functional groups in a molecule with IR spectroscopy.
IR spectroscopy
This form of spectroscopy sends IR radiation towards a sample to measure its absorbance. Absorbance is related to the
type of bond in a molecule.
visible energy
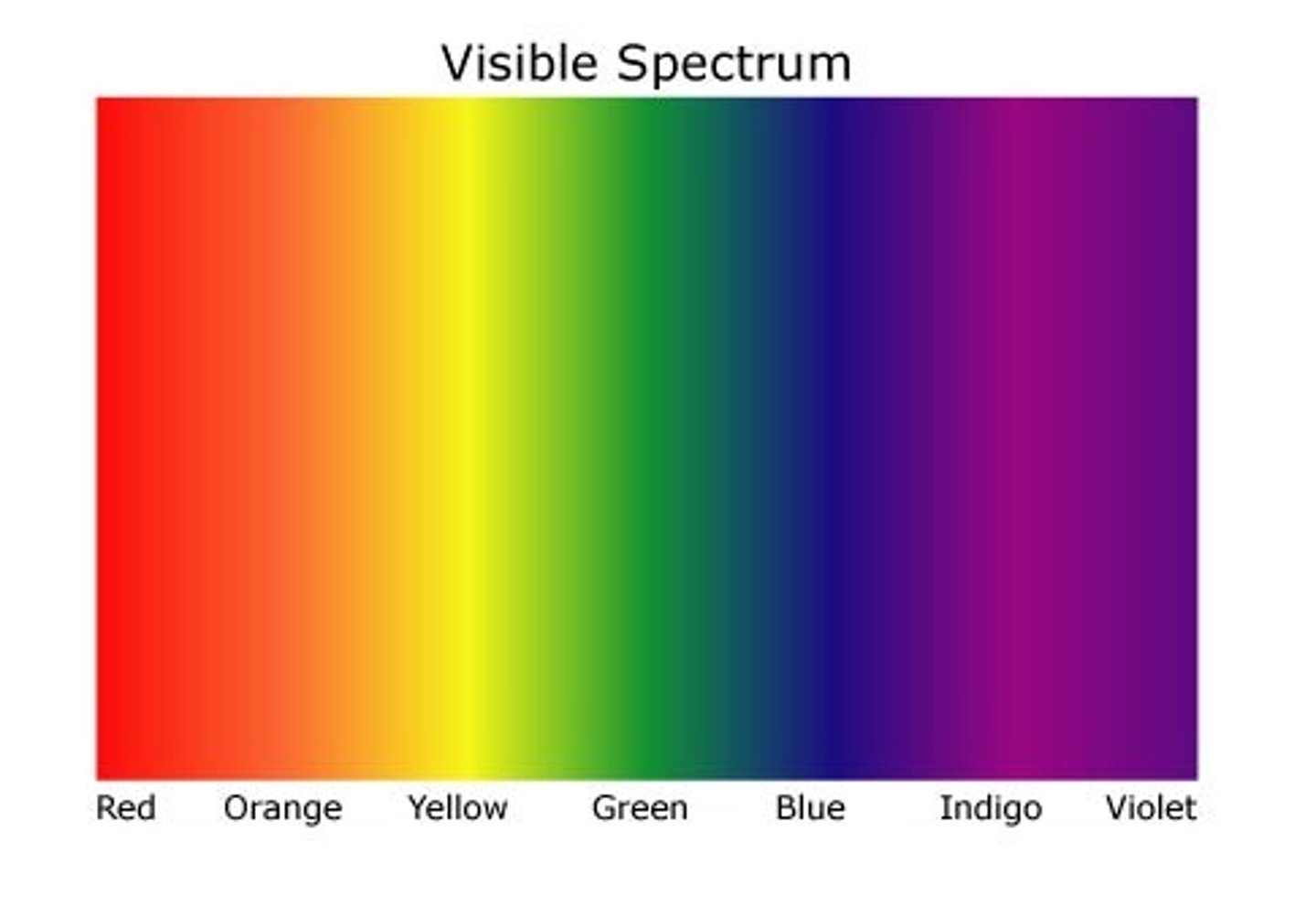
This form of EM energy has wavelengths from around 400 nm to 750 nm, and frequencies around 750 THz to 400 THz. This form of EM radiation electronically excites molecules. It is also used in UV-Vis spectroscopy.
UV energy
This form of EM energy has wavelengths from around 10 nm to 400 nm, and frequencies from 30 PHz to 750 THz. This form of EM radiation excites molecules and can break molecular bonds. It is also used in UV-Vis spectroscopy.
UV-Vis spectroscopy
This analytical technique uses UV-Vis radiation to measure absorbance. The UV-Vis radiation electronically excites the molecules, which raises them to an excited state. During this transition, the absorbance is measured.
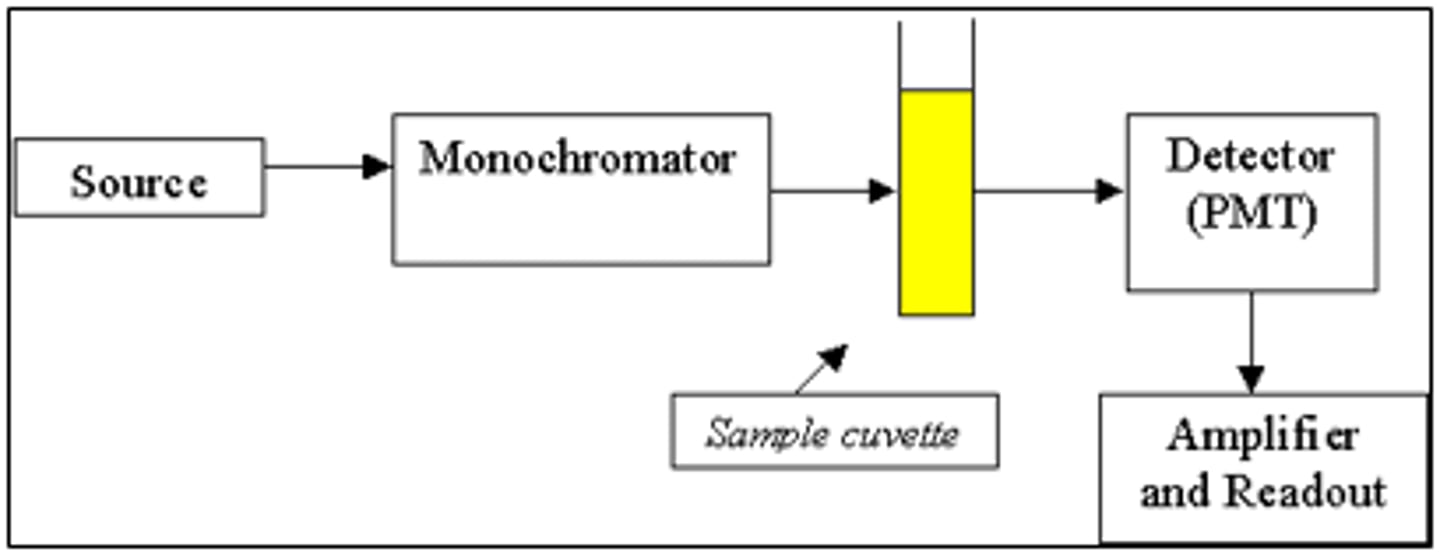
UV-Vis: Step 1
In the first step of UV-Vis spectroscopy, a cuvette is filled with the sample.
cuvette
A plastic-like cylinder which is used for UV-VIs spectroscopy. It is meant to hold the sample for the UV-Vis radiation to pass through.
UV-Vis: Step 2
In the second step of UV-Vis spectroscopy, the cuvette is placed in the spectrophotometer and the absorbance is measured across the desired wavelength range.
UV-Vis: Step 3
In the third step of UV-Vis spectroscopy, the maximum absorbance wavelength is determined.