Chapter 6- Intermolecular forces
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
explain the electron-pair repulsion theory?
electron pairs surrounding a central atom determine the shape of the molecule or ion
the electron pairs repel one another so that they are arranged as far apart as possible
the arrangement of electron pairs minimises repulsion and thus holds the bonded atoms in a definite shape
different numbers of electron pairs result in different shapes
represent the molecule methane in 3 dimensions
four bonded pairs of electrons around the central carbon atom
the 4 electron pairs repel each other as far apart as possible in three-dimensional space
this results in a tetrahedral shape with 4 equal H-C-H bond angles of 109.5
what are intermolecular forces?
weak interactions between dipoles of different molecules
what are the 3 categories of intermolecular forces?
induced dipole-dipole interactions (london forces)
permanent dipole-dipole interactions
hydrogen bonding
induced dipole-dipole?
weakest type of intermolecular interactions
permanent dipole-dipole interactions?
stronger than induced dipoles-dipole but weaker than hydrogen bonds
hydrogen bonds?
strongest form of intermolecular forces
they are still much weaker than INTRAmolecular forces (e.g- covalent bonds)
difference between intermolecular forces and covalent bonds?
intermolecular forces: largely responsible for physical properties such as melting and boiling points
covalent bonds: determine the identity and chemical reactions of molecules
what are the london dispersion forces (induced dipole-dipole interactions)?
weak intermolecular forces that exist between all molecular substances and noble gases
they do not occur in ionic substances
they act between induced dipoles in different molecules
*describe how van der waals forces arise!?
uneven distribution of electrons/ movement of electrons
causes an instantaneous dipole/temporary dipole in a molecule
this then causes induced dipoles in neighbouring molecules
temporary dipoles?
the electron clouds around molecules are constantly in motion
one moment, the electron density can be on one side of the molecule
the next, it can be somewhere totally different
this is called a temporary dipole, there are partial charges, but they change very rapidly
image of london forces?
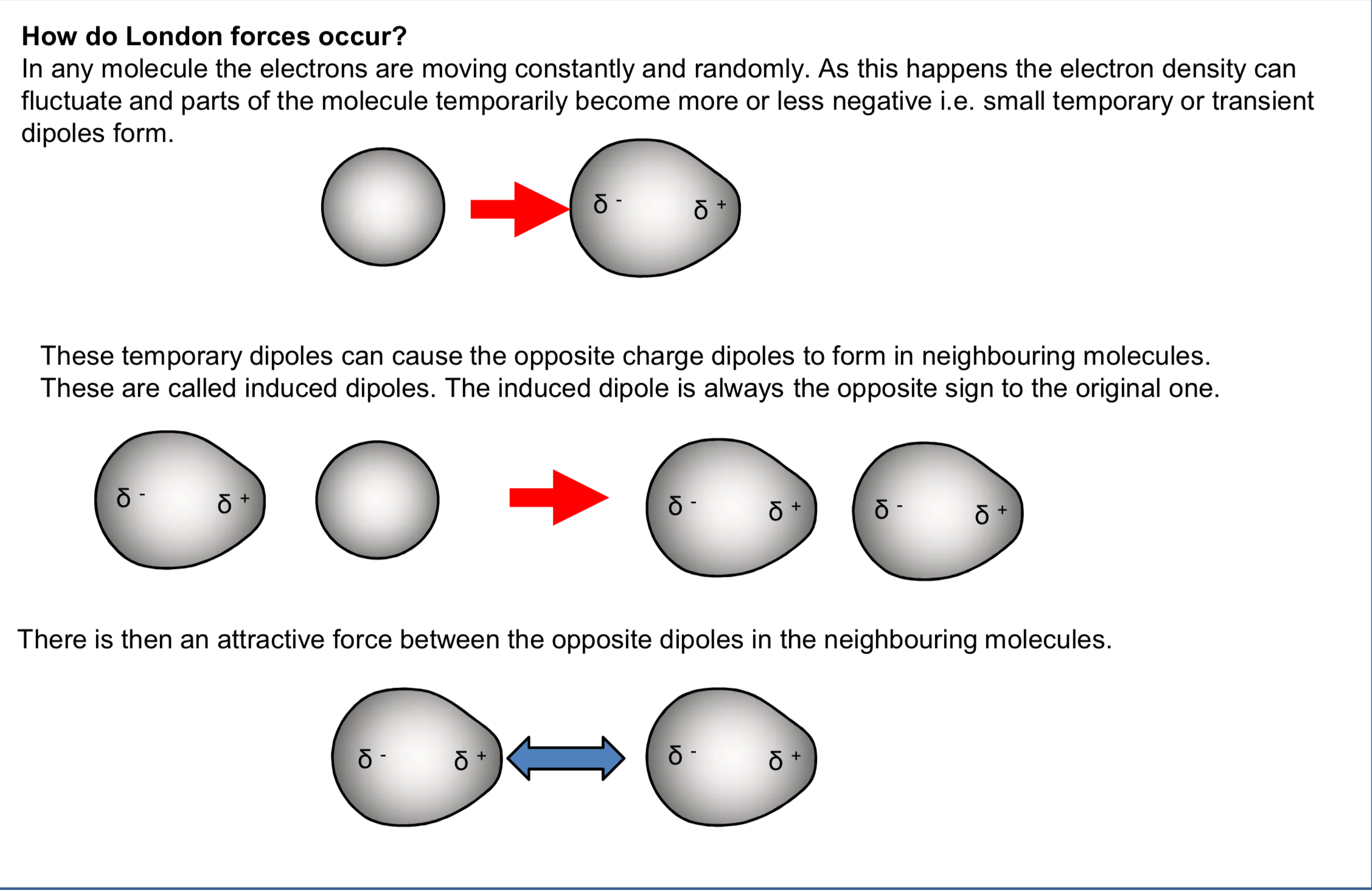
induced dipoles?
if one molecule has a temporary dipole, its partial charges will exert a force on nearby molecules
the partial charge of one molecule can push away the electrons in another, or attract them towards it
this means that temporary dipoles will induce dipoles in nearby molecules
what happens once a dipole has been induced?
it will be attracted to the initial dipole
this is called an induced dipole interaction
what does the strength of london dispersion forces depend on?
the number of electrons in a molecule
this is why london dispersion forces are not all the same strength
question: why does the boiling points of the halogens down group 7 increase?
number of electrons increases in the bigger molecules
this causes an increase in the size of induced dipole-dipole interactions between molecules
this is why iodine is a solid, whereas chlorine is a gas
question: why does the boiling points of the alkane homologous series increase?
the number of electrons increases in the bigger molecules
this causes an increase in the size of the induced dipole-dipole interactions between molecules
what else has an effect on the size of the induced dipole-dipole interactions?
the shape of the molecule
example of shape of molecule having an effect on the dipole interactions?
long chain alkanes have a larger surface area of contact between molecules for induced dipole-dipole interactions to form
compared to spherical branched alkanes
therefore, they have induced dipole-dipole interactions
why will molecules with more electrons have stronger london dispersion forces?
because they have lots of electrons
this is because they will have larger fluctuations in electron density
this then leads to larger temporary dipoles and stronger dipole-dipole interactions
where do permanent dipole-dipole interactions exist between?
they exist between 2 permanently polar molecules
permanent dipole image?
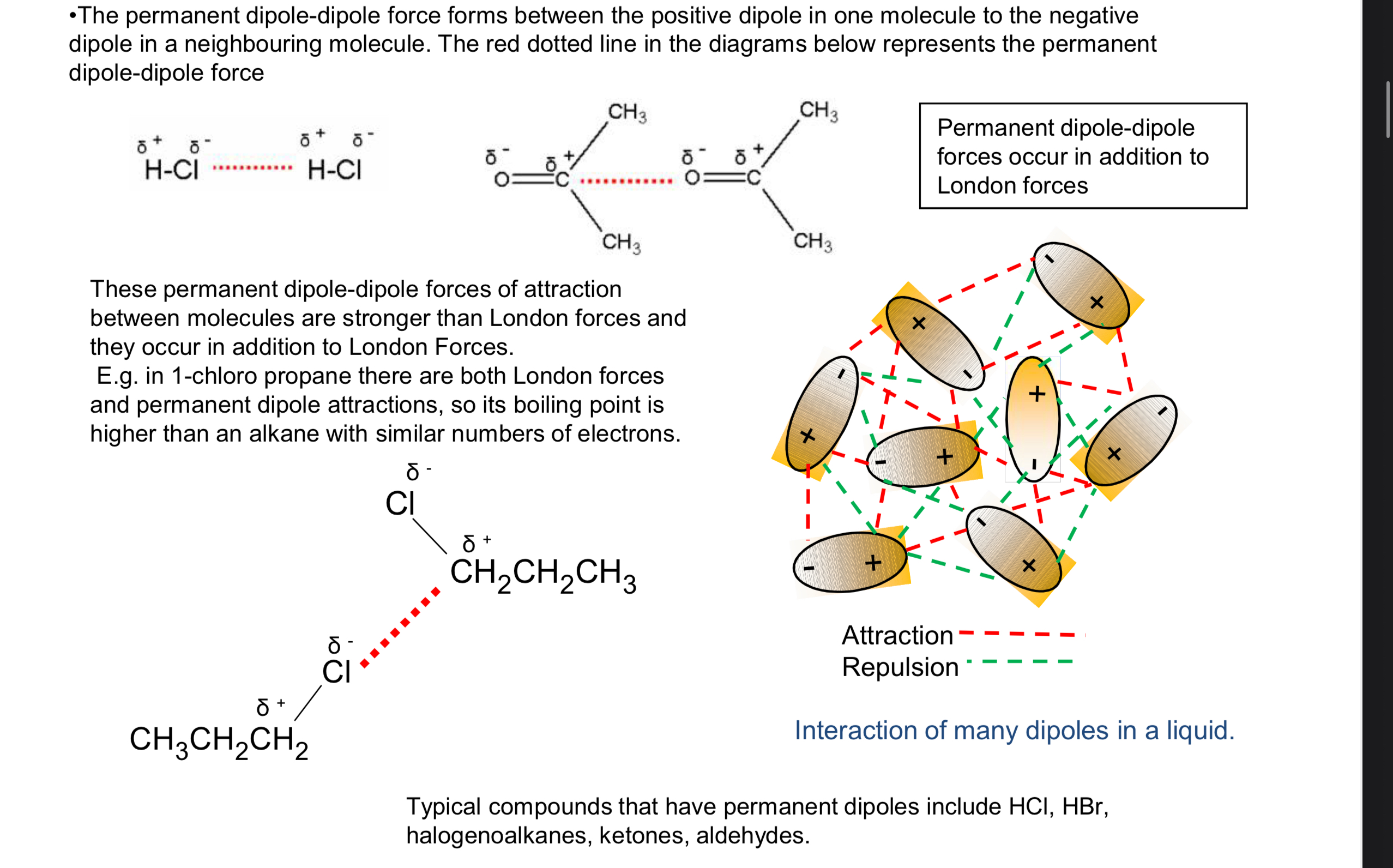
what symbols do permanent dipole-dipole interactions have?
they have a delta- on one side and a delta + on the other side
the delta + of one molecule will attract the delta - of another molecule
where are hydrogen bonds found?
when you have a hydrogen atom bonded to either oxygen, nitrogen or fluorine
that hydrogen atom will form a strong permanent dipole-dipole interaction with another oxygen, nitrogen or fluorine atom
examples of liquids with hydrogen bonds?
water
hydrogen fluoride
ammonia
why do hydrogen bonds form?
when hydrogen is bonded to an extremely electronegative element, it develops a strong delta + charge
hydrogen is a very small atom, so it has a high charge density in this situation
this allows it to form a strong bond with any highly delta - charged atom
why do H2O, NH3 and HF have anomalously high boiling points?
because of the hydrogen bonding between the molecules in addition to their london forces
the additional forces require more energy to break and therefore, they have higher boiling points
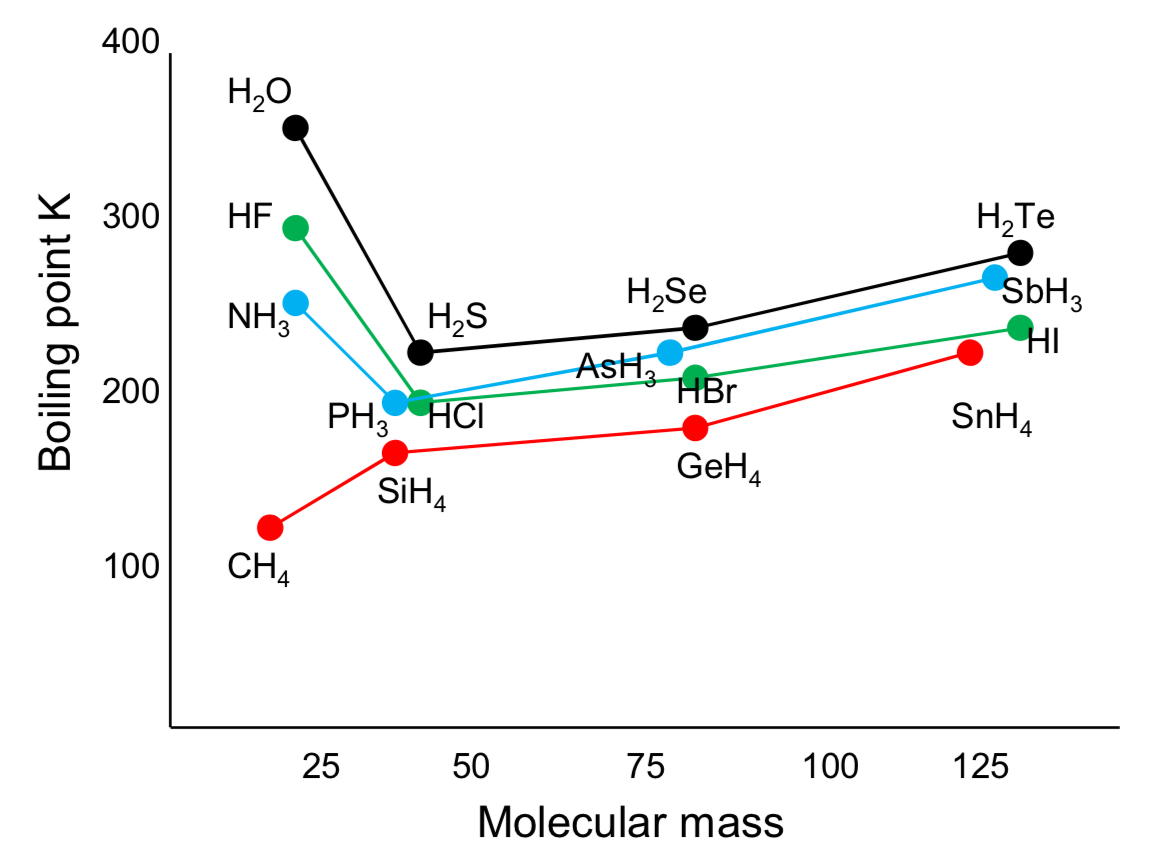
why is there a general increase between the molecules?
because there are more electrons in the bigger molecules and therefore, they have stronger london forces
properties of compounds with hydrogen bonding?
have higher boiling points compared to compounds with other types of intermolecular forces
tend to be soluble in other compounds with hydrogen bonds
they have higher viscosity
the stronger the hydrogen bonding, the more viscous the liquid
they have higher surface tension
what acronym is used to remember how to draw hydrogen bonding?
P- partial charges
H- hydrogen bond
I- in line
L- lone pair
draw the hydrogen bonding between methanol molecules
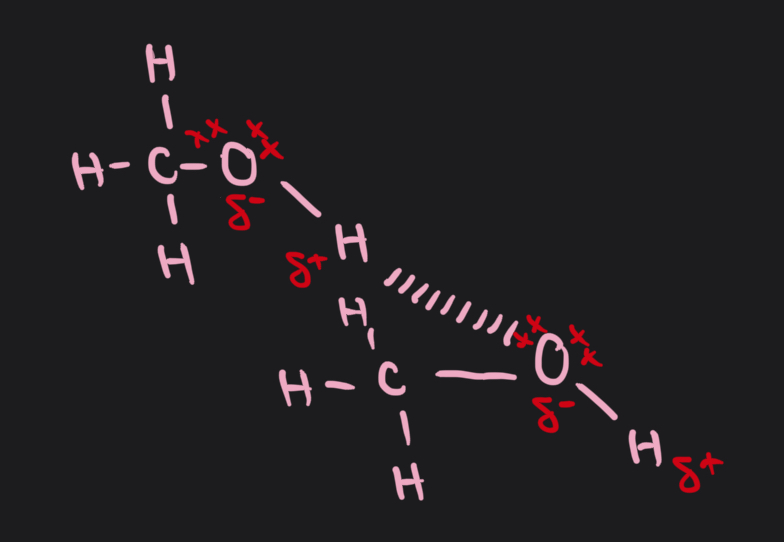
why is ice less dense than water?
hydrogen bonds hold water molecules apart in an open lattice structure
the water molecules in ice are further apart than in water
solid ice is less dense than liquid water, and floats