Chap 15A - Arenes (Benzene)
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Describe nomenclature for benzene
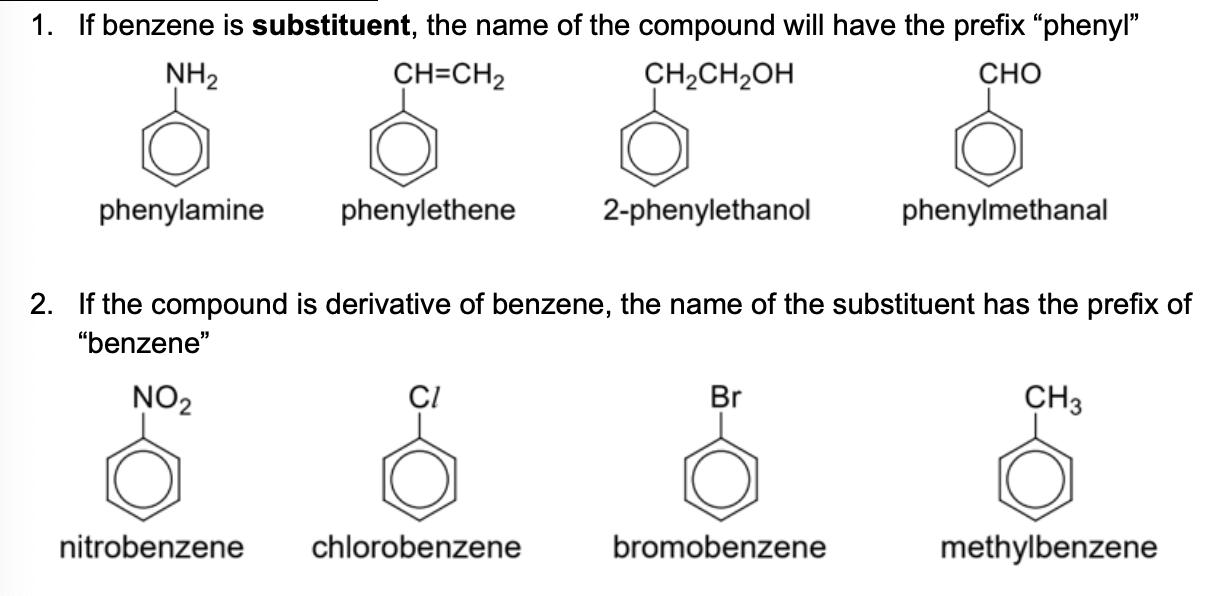
State the special names
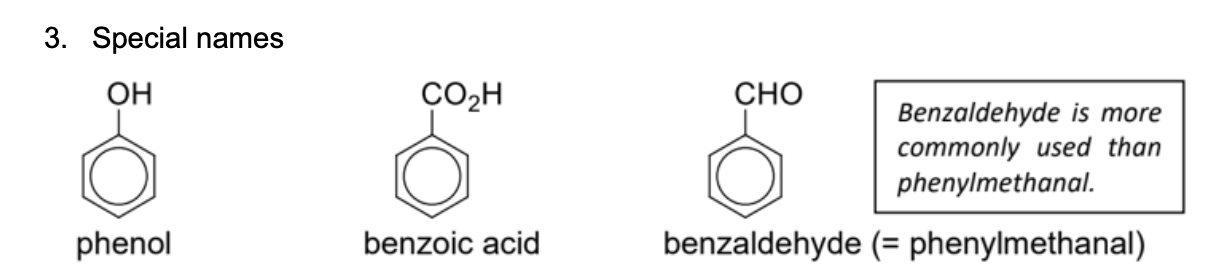

Name these
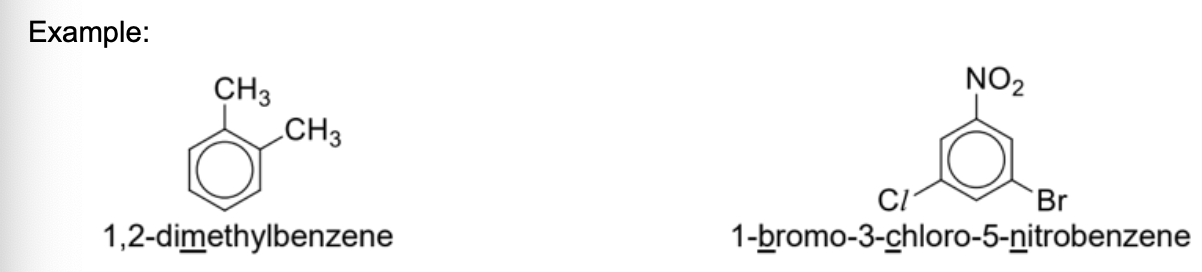
Describe mp and bp of arenes
Arenes are liquids or low melting point solids with characteristic ‘aromatic’ odours
Their vapours are toxic (don’t inhale them)
Eg. Benzene is a colourless liquid (boiling point 80C, melting point 5.5C) and continued inhalation of its vapour can induce anaemia and leukaemia
Eg. Methylbenzene is also a colourless liquid (with a higher boiling point 111C)
Bp of arenes increase with increase in relative Mr due to increase in number of electrons leading to stronger id-id interactions that require more energy to overcome
Mp trend is irregular as it depends on molecular symmetry
Describe solubility of arenes
Arenes are soluble in organic solvents such as CCl4
Both benzene and methylbenzene are useful solvents
Since the fumes of methylbenzene are considerably less toxic than those of benzene, it is preferable instead of benzene
Arenes are insoluble in polar solvents such as water and are less dense than water
Arenes are non-conductors of electricity
Arenes burn with a smoky and luminous flame due to high carbon content (C:H ratio is close to one)
Describe formation of pi electron cloud in benzene
Each carbon atom in benzene has an unhybridised 2p orbital that is perpendicular to the hexagonal plane of carbon atoms
Each of the six 2p unhybridised orbital contains an electron
Since these six 2p orbitals are parallel to one another, each of the 2p orbital can overlap side-on and equally with the adjacent two 2p orbitals to form bonds
The side-on overlap of the 2p orbitals results in a doughnut-shaped delocalised electron cloud above and below the hexagonal plane of carbon atoms
Explain why benzene exists as a resonance hybrid
Due to delocalisation of the electrons, the benzene molecule exists as a resonance hybrid of the resonance structures (I) and (II)
NOTE: resonance structures (I) and (II) do NOT exist
The delocalisation conferred extra stability to benzene -> benzene is resonance-stabilised
Hexagon = six carbon atoms arranged in a hexagonal ring
Circle = delocalised six electrons
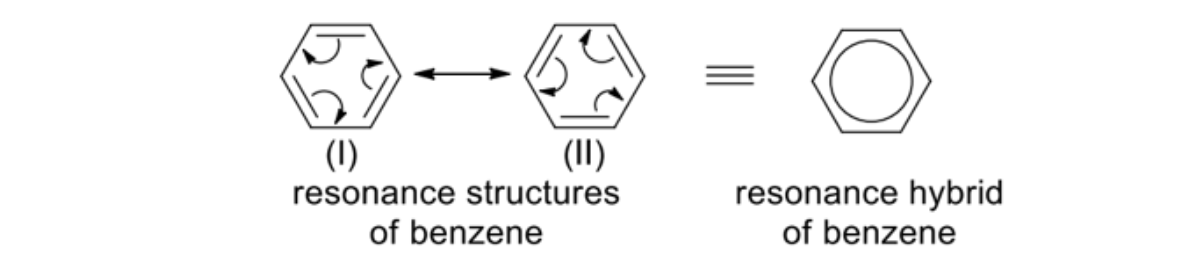
Explain how benzene same C-C bond length show it is a resonance hybrid
Benzene has the same carbon-carbon bond lengths of 0.139 nm which are intermediate in length between C–C bond and C=C bond
C-C bond in benzene is stronger than C–C bond but weaker than C=C bond
The unhybridised 2p orbital of each carbon atom can overlap sideways and equally with the adjacent two 2p orbitals
Explain how benzene heat of hydrogenation show it is a resonance hybrid
Heat of hydrogenation (heat evolved when 1 mol of unsaturated compound is hydrogenated) of benzene has a lower magnitude than expected
Benzene evolves 151 kJ mol–1 less energy than predicted -> benzene is more stable by 151 kJ mol–1 than expected -> benzene is resonance–stabilised with resonance energy = 151 kJ mol–1
Explain why benzene undergoes substitution reactions readily rather than addition reactions
Preserve delocalised pi electron cloud which forms a resonance stabalised-ring structure
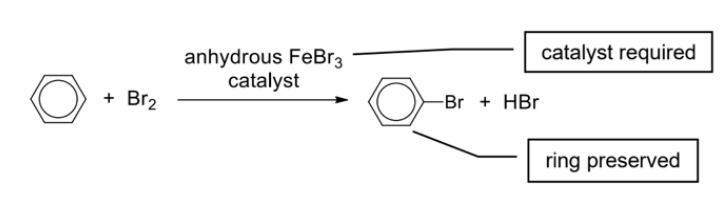
Describe benzene substitution reaction with Br + general reaction
Eg. Unlike alkenes, benzene does not decolourise orange-red liquid bromine: It undergoes substitution reaction with bromine in the presence of anhydrous FeBr3 catalyst
Describe electrophilic substitution of arenes
Eg. Halogenation, Nitration, Friedel-Crafts alkylation
Benzene possesses high electron density and is a source of electrons -> attacked by electrophiles
Resonance stabilisation of benzene structure due to the delocalisation of electrons makes the benzene ring less reactive towards electrophiles compared to the C=C bond in alkenes -> benzene can only be attacked by strong electrophiles (Eg. Br+)
Draw electrophilic substitution mechanism
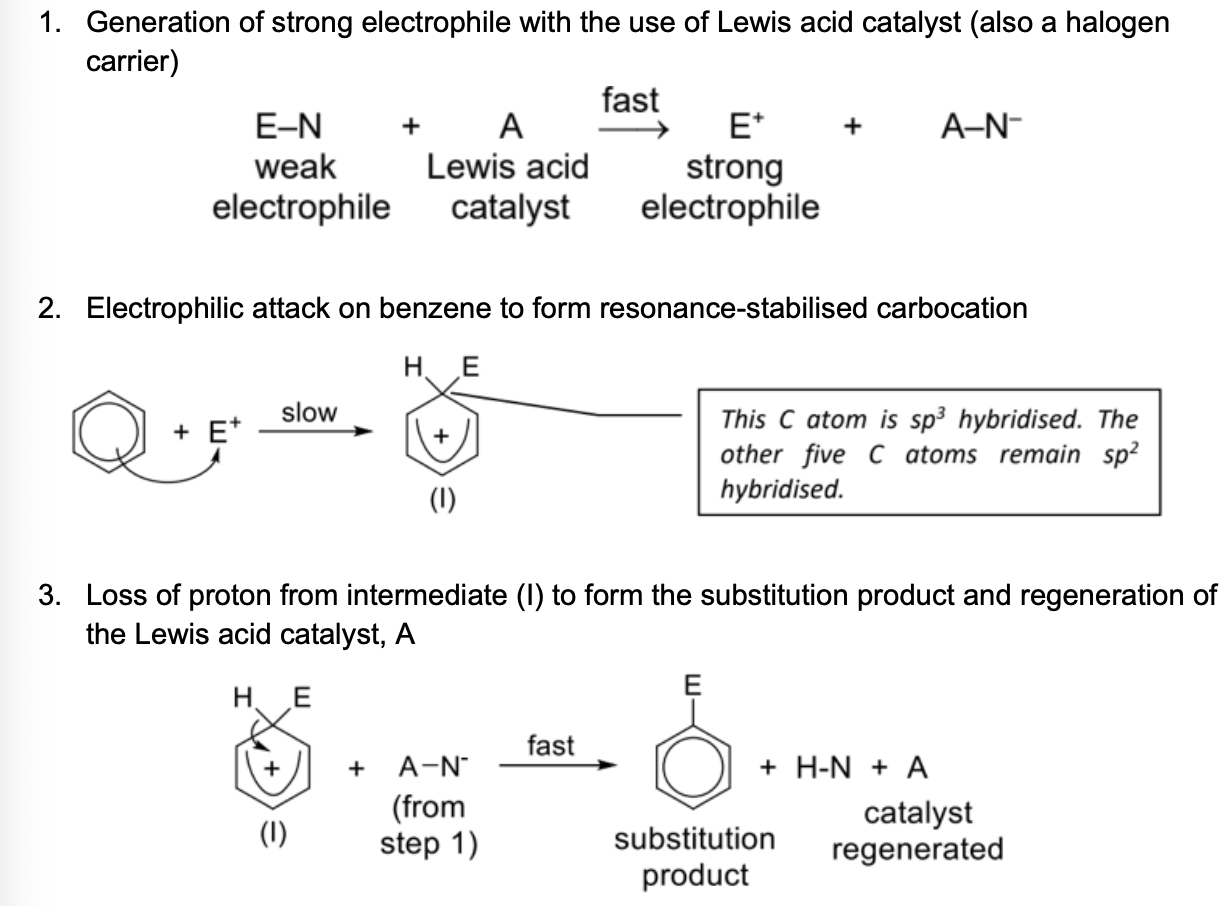
Decribe intermediate formed in electrophilic substitution
NOTE: Benzene loses a proton in the process
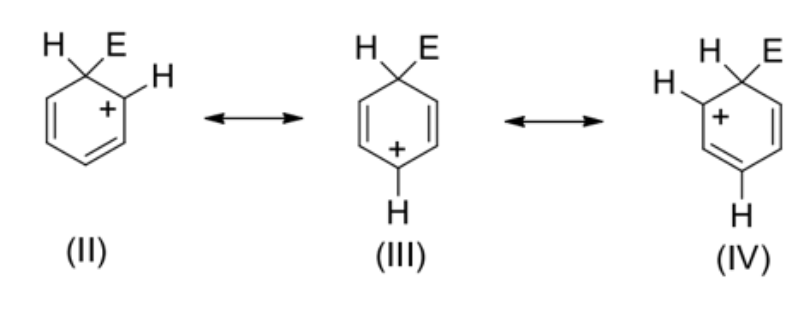
In (I), the organic intermediate, only five orbitals are overlapping and the positive charge is delocalised over five carbon atoms
(I) is a resonance–stabilised carbocation and is a resonance hybrid of (II), (III) and (IV)