Chemistry - Forces of Attraction
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
forces of attraction
the interactions that occur between particles and cause them to be drawn to one another — creating a chemical bond
What are the main types of forces of attraction? (4)
ionic bonds, covalent bonds, metallic bonds and van der Waal forces
Describe giant substances in terms of their volatility and solubility (2)
Giant substances are relatively involatile due to the strong forces of attraction between particles
Giant substance can be soluble in polar solvents if their dipoles are partial
Describe molecular substances in terms of their volatility and solubility (2)
Molecular substances are relatively volatile due to the weak forces attraction between molecules
Molecules lack significant dipoles between each other and have are weakly attracted to each other and therefore polar solvents like water cannot interact with them whereas they are soluble in non-polar solvents due to dispersion forces
Describe metals in terms of their volatility and solubility (2)
Metals are relatively involatile due to the strong forces of attraction between the particles in their lattice
These substance are insoluble in both polar and non-polar substances as their atoms can’t interact with a solvent due to their high density
Describe ionic bonding
the instance in which a metal atom transfers its electron to a non-metal atom and ionizes into a positive charged particle while the non-metal ionizes into a negatively charged particle which are held together by electrostatic forces in a three dimensional lattice fueled by the electronegativity of non-metals in which attractive forces interact with charged ions in multiple directions
Describe covalent bonding
the instance in which atoms approach each other and an equilibrium is achieved between the repulsion of their electron clouds and their nuclei resisting each other in the atoms reaching a position of least potential energy where they are sharing electrons in a resultant molecular orbital and a chemical bond persists until sufficient energy is provided to disrupt this dynamic — achieving stability
Describe the relationship between the electronegativity of two atoms and their polarity in a covalent bond
If the electronegativity values of the two atoms in a covalent bond are very similar, they are non-polar. If they are not the same, they are considered polar.
sigma bond
covalent bond formed by the overlap of atomic orbitals alongside a line between the two nuclei
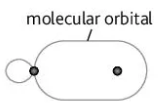
Describe this image
sigma bond between a s and p orbital
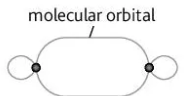
Describe this image
sigma bond between two p orbitals
pi bond
bond formed from the sideway overlap of p orbitals irrespective of the symmetry about a line joining the two nuclei
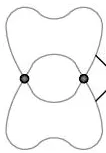
Describe this image
pi bond
Describe the relationship between the type of covalent bond and the amount of bonds between atoms (3/2)
In a single bond, there is one singular sigma bond.
In a double bond, there is one sigma bond and a pi bond.
In a triple bond, there is one sigma bond and two pi bonds.
Sigma bonds will always form first after orbitals overlap end-to-end. After which, pi bonds will form after orbitals interact with each other sideways to form multiple bonds.
coordinate/dative bonding
the instance in which an atom donates a lone pair of electrons to an atom in a covalent bond
metallic bonding
instance in which metals achieve stability via orbitals in metal atoms overlapping to form a band structure where a sea of delocalized electrons can travel throughout the subsequent lattice formed in the metal and hold the metal ions together through electrostatic forces
Describe chemical bonding in terms of orbital overlap
In chemical bonding, two atoms approach each other due to the electrostatic forces of attraction between each other’s nuclei and electrons until an equilibrium is reached after which two molecular orbitals are formed — the bonding orbital and antibonding orbital.
Electrons occupy the bonding orbital as it is closer to the nucleus and has lower energy while electrons do not exist within the antibonding orbital excepts when excited because it is further from the nucleus and serves as a region of repulsion due to its low electron density and role in increasing nucleus-nucleus repulsion.
The net force between the bonding orbital and antibonding orbital keep the molecule stable and the energy difference between the two exists as the band gap.
Describe metallic bonding in terms of orbital overlap
Due to the large atomic radius of metal atoms, they tend to overlap and their low electronegativity allows their electrons to delocalize forming a sea of electrons which oscillate between the two atoms — reducing the energy of the system of two orbitals.
As the energy between the two decreases, the metal atoms approach each other more closely however due to low resistance from the nucleus and rampant electrostatic forces between the delocalized electrons and subsequently formed cations repulsion reduces as electrons evenly distribute between the two orbitals until the bond becomes stable, leading to a complete overlap of the valence and conductive bands
Explain metallic conductivity using energy bands
The overlap of the valence band and conductive band affords electrons enough energy to move across the structure and carry heat and electricity
intermolecular forces
forces of attraction which arise as a result of the attraction between the dipoles of neighbouring molecules
van der Waal forces
umbrella term used to define and categorize the types of attraction between all molecules
Permanent dipole-dipole forces
Keesom forces
weak intermolecular forces that arise between permanently polar molecules when their oppositely charged poles produce electrostatic forces
induced dipole-induced dipole forces
London dispersion forces
interaction in which electrons in a neutral molecule randomly move within it and make it slightly polar whilst its nearby another molecule and a dipole is induced creating a momentary attractive force between them
dipole-induced dipole forces
London dispersion forces
interaction in which a polarized molecule with a charge approaches a neutral molecule or the neutral position of another identical molecule and the charge either repels or attracts nearby electrons leading to a momentary bond
hydrogen bond
instance in which a hydrogen atom covalently bonded to a highly electronegative atom becomes so electron deficient that it gains the ability to interact with a lone pair of electrons on another highly electronegative atom
Describe how hydrogen bonds affect the miscibility, solubility and volatility of water
Substances with hydrogen bonds mix well with other polar substances
Polar molecules capable of hydrogen bonds dissolve easily in water because they can form hydrogen bonds with water molecules
Water has a high melting/boiling point for its size as its hydrogen bonds increase the amount of energy needed to break the bonds between molecules
Describe the role hydrogen bonds play in water’s maximum density
In the case of water, it is at its densest at 4 degrees Celsius due to its arrangement as a liquid in which the increase in thermal energy allows water molecules to exist in continuous motions and only form non-rigid, transient hydrogen bonds with each other and can pack closely together unlike ice molecules which are held in a rigid, open, hexagonal lattice to maximize bonding
VSEPR (2)
valence shell electron pair repulsion theory
model used in chemistry to predict the 3D geometry of simple individual molecules from the number of electron pairs surrounding their central atoms
What are the laws of VSEPR? (3)
Pairs of electrons in the outer shells of the atoms in a molecule repel each other and move as far apart as possible — minimizing repulsive forces in the molecule
Repulsion between lone pairs and lone pairs is greater than repulsion between lone pairs and bond pairs of electrons
Repulsion between lone pairs and bond pairs is greater than repulsion between bond pairs and bond pairs of electrons
Describe the shapes of molecules composed solely of bonding pairs (5)
linear — two bonding pairs with bond angles of 180°
trigonal planar — three bonding pairs with bond angles of 120°
tetrahedral — four bonding pairs arrange themselves in space at maximum distance apart with bond angles of 109.5°
trigonal bipyramidal - five bonding pairs arrange themselves in space at maximum distance apart with three triagonal plane bonds with angles of 120° and two atoms forming linear angles of 180° to each other
octahedral — six bonding pairs arrange themselves with four atoms with bond angles of 90° and two atoms forming linear angles of 180° to each other
Describe the shapes of molecules composed of lone pairs and bonding pairs (4)
trigonal planar — two bonding pairs and a lone pair bond angles less than 120°
tetrahedral — three bonding pairs and a lone pair OR two bond pairs and two lone pairs with bond angles of less than 109.5°
octahedral — four bonding pairs and two lone pairs with square planar bond angles of 90° and linear bond angles of 180°
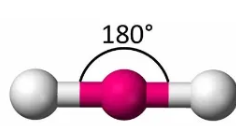
Describe the shape of this molecule
linear with two bonding pairs
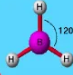
Describe the shape of this molecule
trigonal planar with three bonding pairs
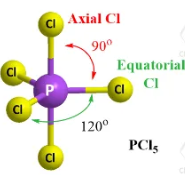
Describe the shape of this molecule
trigonal bipyramidal with five bonding pairs
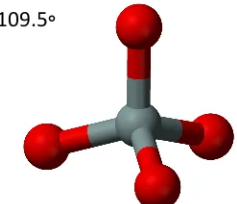
Describe the shape of this molecule
tetrahedral with four bonding pairs
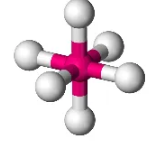
Describe the shape of this molecule
octahedral with six bonding pairs
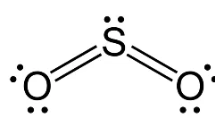
Describe the shape of this molecule
trigonal planar with two bond pairs and a lone pair
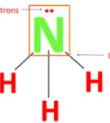
Describe the shape of this molecule
tetrahedral with three bond pairs and a lone pair
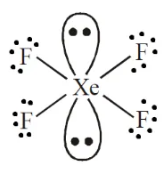
Describe the shape of this molecules
octahedral with four bonding pairs and two lone pairs
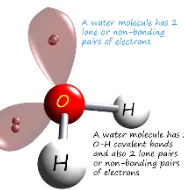
Describe the shape of this molecule
tetrahedral with two bonding pairs and two lone pairs
hybridization
a conceptual instance that theorizes that orbitals combine in order to bond more effectively
Describe the hybridization in ethane
In ethane, each C atom forms four sp3 orbitals. Two of these orbitals overlap and the remaining six overlap with the hydrogen atoms in the —CH3 groups
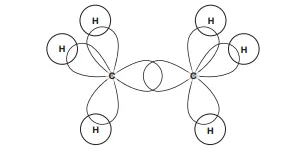
Describe this image
orbital diagram of ethane
Describe hybridization in ethene
In ethene, each C atom forms three sp2 orbitals that lie 120° apart from each other. These orbitals form two sigma bonds with two hydrogen atoms and one with a carbon atom. A pi bond is formed between the C atoms as well from the overlap of their unhybridized p orbitals
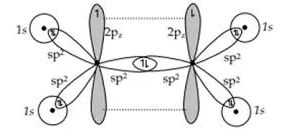
Describe this image
Orbital diagram of ethene
resonance
instance in which the bonding present in a molecule cannot be expressed in a singular way
Describe hybridization and resonance in benzene (2)
In benzene, the six carbon atoms form a hexagon with three localized sp2 orbitals (one to each hydrogen atom and two to other carbon atoms)
The unhybridized p orbitals overlap to form a delocalized system of pi bonds in which electrons can freely move and hence change the amount of bonds present amongst atoms
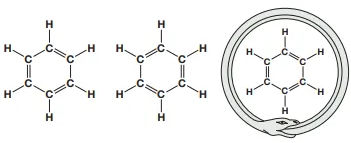
Describe this image
Possible structures of benzene
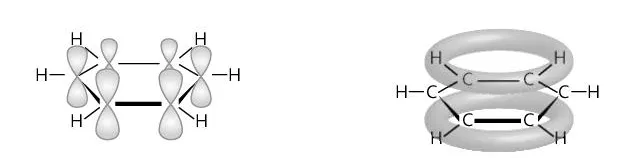
Describe this image
orbital overlap of benzene