P3 - Particles
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
What is the equation for density?
P = m/v
How do you find the volume of a regular object?
length x width x height
What piece of equipment can be used to find the mass of an object?
Top pan balance
How do you find the volume of a irregular object?
Using a eureka can or a measuring cylinder
How can you use a eureka can to find the volume of an irregular object?
Fill the eureka can up to the spout
Using string, submerge object and wait for all water to be displaced out into a beaker
Pour water into a measuring cylinder to measure the volume
Internal energy
The sum of kinetic energy and potential energy of all particles in a substance
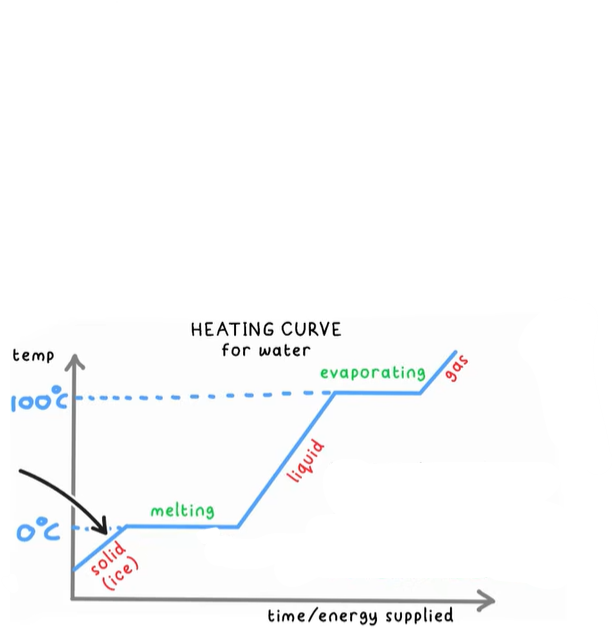
What is happening to the particles if they are increasing in temp?
They are gaining kinetic energy
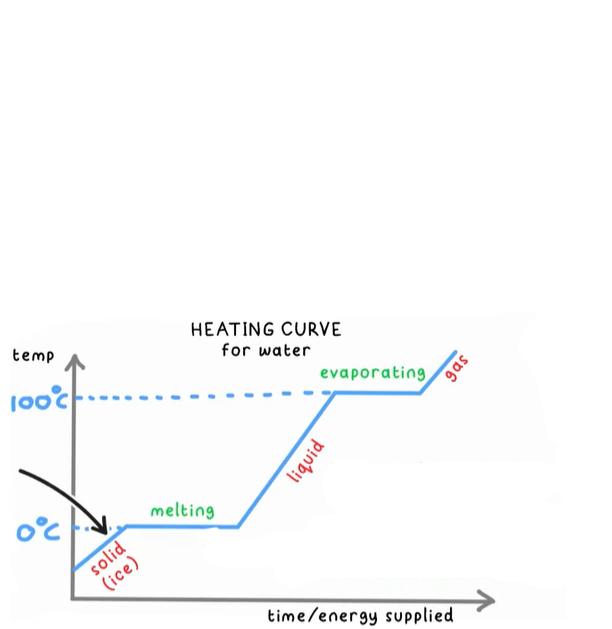
What is happening while the temperature remains constant?
The substance is changing state, they are gaining potential energy
What is energy being used for during a change of state?
To overcome the electrostatic forces of attraction between the particles
Latent heat
The energy/heat required to change the state of a substance
What is the equation to calculate the change in thermal energy?
Change in E = mc x change in t
Specific latent heat
The energy required to change the state of 1kg of a substance
What is the equation to calculate latent heat?
E = mL
What is gas pressure a result of?
Particles colliding with the walls of its container, exerting a force outwards
How does heating a gas cause the pressure to increase?
By heating a gas, you increase the particles kinetic energy, meaning they collide more frequently and with greater force so there is an increase in pressure, assuming the volume stays constant