BSC 2010
1/120
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
121 Terms
Attributes of living organisms…
A common set of chemical parts (nucleic acids such as DNA and amino acids that make up proteins)
All composed of similar structures (such as cells enclosed in membranes)
Depend on interactions between structurally complex parts to maintain the living state
Contain genetic information that codes for proteins
Convert molecules obtained from their environment into new biological molecules
Extract energy from the environment and use it to carry out life functions
Replicate their genetic information in the same way
Have a fundamental set of genes
Evolve through gradual changes in their genetic information (mutations due to external or internal forces)
Viruses
Contain genetic information, mutate, and evolve
Considered living things by most biologists, but they are not composed of cells and cannot carry out functions on their own
Nucleic acids
molecules that can reproduce themselves and contain the information for synthesis of proteins
Prokaryotes
first organisms, made up of single cells containing genetic material and other structures enclosed in a membrane
Photosynthesis
a set of chemical reactions that transforms sunlight energy into chemical bond energy
Aerobic metabolism
a set of chemical reactions that releases energy from molecules using O2
Anaerobic metabolism
set of reactions that extracts energy without using O2
Eukaryotes
cells with a nucleus
Endosymbiosis
(living inside another): a process by which certain organelles were created. Smaller cells were ingested by larger cells
Ex. Mitochondria, Chloroplasts
Cellular specialization:
cells in groups are able to specialize (perform different functions)
Some cells might specialize in reproduction, nutrient absorption, etc.
Two kinds of research
Discovery-based research
Hypothesis-driven research
Discovery-based research
analysis of lots of collected data to find new patterns and generate questions or ideas
No endpoint/question to the research
Hypothesis-driven research
testing answers to a specific question using the scientific method
Scientific Method
Make observations
Ask a question, usually using why or how?
Formulate possible answers to the question (alternative hypotheses)
Make predictions: what will be true if my prediction is correct?
Design and conduct an experiment
Use statistical tests to calculate probability of observed data if hypothesis is correct
a. If results support hypothesis, then repeat the experiment and ask new questions
b. If results do not support the hypothesis, reexamine the experiment for uncontrolled variables and conduct the experiment again. If results are still not supporting hypothesis, then revise the hypothesis
All living and nonliving matter is composed of…
atoms!
Element (what is an element, how many, which are most abundant)
substance that consists of one type of atom
92 naturally occurring elements
CHON (carbon, hydrogen, oxygen, and nitrogen) are the most abundant elements in living systems
Structure of an atom
Protons (+)
Neutrons (neutral = no charge)
Electrons (-)
Atomic mass is measured in
measured in daltons/amu (1 dalton = mass of 1 proton or neutron)
Mass of electrons is so low that it is ignored when calculating the atomic mass
Atomic number
elements are identified by the number of protons (atomic number)
Isotope
versions of elements with different numbers of neutrons
Mass number
total number of protons and neutrons
Columns on periodic table
have the same number of electrons in their valence shell (similar reactive properties)
Orbitals
Every shell has an s orbital (one pair of electrons)
Shells after the first shell also have a p orbital (3 pairs of electrons)
Lower level shells have less energy than higher level shells
s orbitals are lower energy than p orbitals
Atoms with unfilled outer shells undergo chemical reactions
Reaction = gaining or losing electrons
Atoms are stable when their valence shell is full
Octet rule
atoms with at least two electron shells form stable bonds when they have 8 electrons in their valence shell
Electronegativity
an atom’s tendency to attract electrons from another atom
More electrons in the outermost shell = higher EN
Electrons closer to the nucleus have higher EN
EN increases from the bottom left of the periodic table (lowest EN) to top right (highest EN)
Ionic bonds
High EN atom steals electron(s) from low EN atom to form charged particles
The force of attraction between these charged particles is called an ionic bond
Cation (positively charged atom)
Anion (negatively charged atom)
Salts: molecules held together by ionic bonds
Covalent bonds
Each atom counts those electrons as part of itself
Covalent bonds are very strong
Occur between similar EN atoms
Nonpolar covalent: equally shared e
Polar covalent: unequally shared e
Dipole-dipole interactions
electrostatic attractions that occur between polar molecules, where the positive end of one molecule is attracted to the negative end of another
Hydrogen bonds
Van der Waals interactions
Random attractions due to movement of electrons creating temporary dipoles
Occurs within all molecules (polar and nonpolar)
Stability of a molecule is closely associated with
its level of energy
Less stable molecules have higher energy and are converted during chemical reactions into lower energy, more stable molecules
Energy
capacity to produce a change
Kinetic energy
energy of movement (includes thermal, sound, and electromagnetic energy
Potential energy
stored energy (includes gravitational, elastic, chemical bond, and nuclear energy)
All changes in the universe involve….
Energy transformation
Laws of thermodynamics
Energy cannot be created or destroyed (Total amount of energy in a closed system remains constant)
With each energy transformation, entropy increases
Entropy: measure of disorder, how spread out energy is, how much of the energy is unusable
Increase in entropy implies that energy is becoming less concentrated and less useable, which is the natural tendency for energy
After each energy transformation, some energy in the system becomes unavailable to do work
Only an input of energy can impose order on a system (ex. hot tea kettle cools down on its own but requires input of energy to be heated)
Second law explains why some reactions occur spontaneously and others do not
If change in entropy is negative, rxn occurs spontaneously
If change in entropy is positive, rxn requires energy
Chemical reaction
occurs when atoms combine or change their bonding partners
Ex: hydrolysis: molecule interacts with water, leading to breakdown into simpler molecules
Hydrolysis
molecule interacts with water, leading to breakdown into simpler molecules
Hydrolysis is exergonic and produces molecules with lower potential energy (stronger bonds)
Free energy
amount of energy available to do work
(ΔG) = total energy change of a reaction
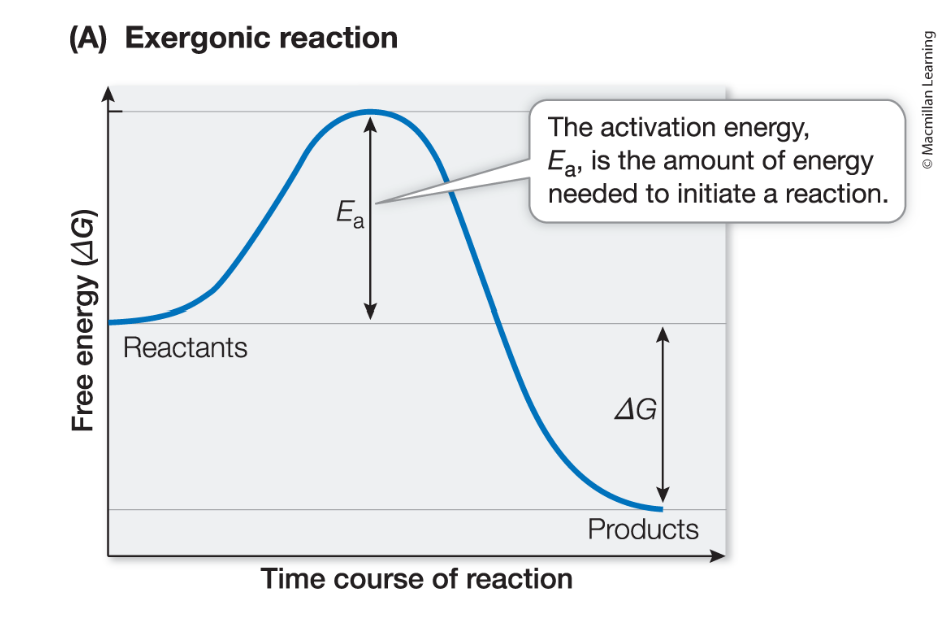
Exergonic reaction
negative ΔG, the reaction releases energy (chemical energy stored in bonds of the products is less than the chemical energy stored in the bonds of reactants)
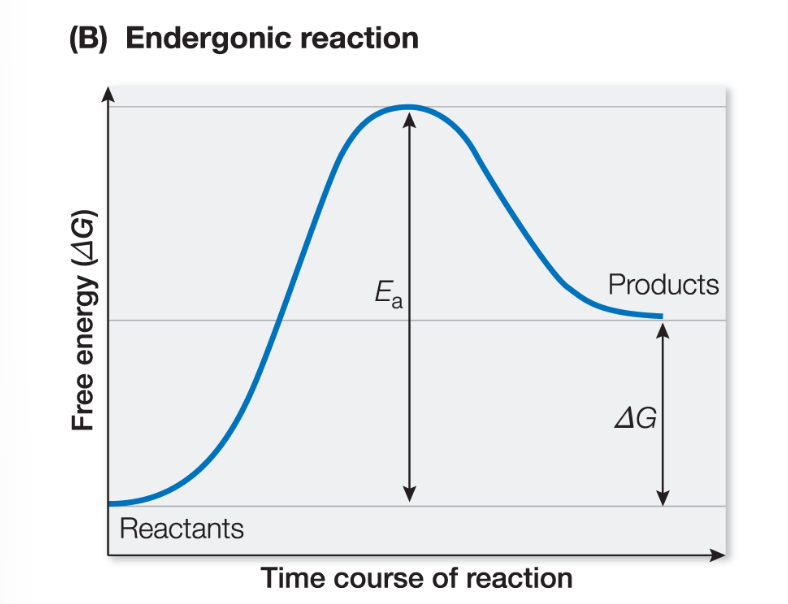
Endergonic reaction
positive ΔG, the reaction requires energy to occur (bonds of product molecules contain more energy than bonds of reactant molecules)
Condensation reaction
two molecules combine to form a larger molecule, producing water as a byproduct
Opposite of hydrolysis
Condensation reactions are endergonic and produce larger molecules with more potential energy (weaker bonds)
Activation energy
the energy that must be supplied for a reaction to begin
Energy to break the covalent bonds of reactants (before products can be formed, releasing energy)
both exergonic (spontaneous) and endergonic (non-spontaneous) require additional energy to initial the reaction
Reaction rate
measures how fast products are made per unit of time
Factors that affect reaction rate
Activation energy
If collision has sufficient energy to reach activation energy, then reaction can occur
Lower activation energy → reaction is more likely to occur
Temperature
Higher temperature increases number of collisions and energy of collisions, making it more likely that collision reaches the activation energy
Concentration
Higher concentration = more likely to collide
Equilibrium
the state in which the rate of forward and reverse reactions is relatively equal and so the relative concentrations of reactants and products will not change
4 basic categories of molecules
Carbohydrates
Lipids
Proteins
Nucleic acids
All of these 4 biomolecules have carbon backbones
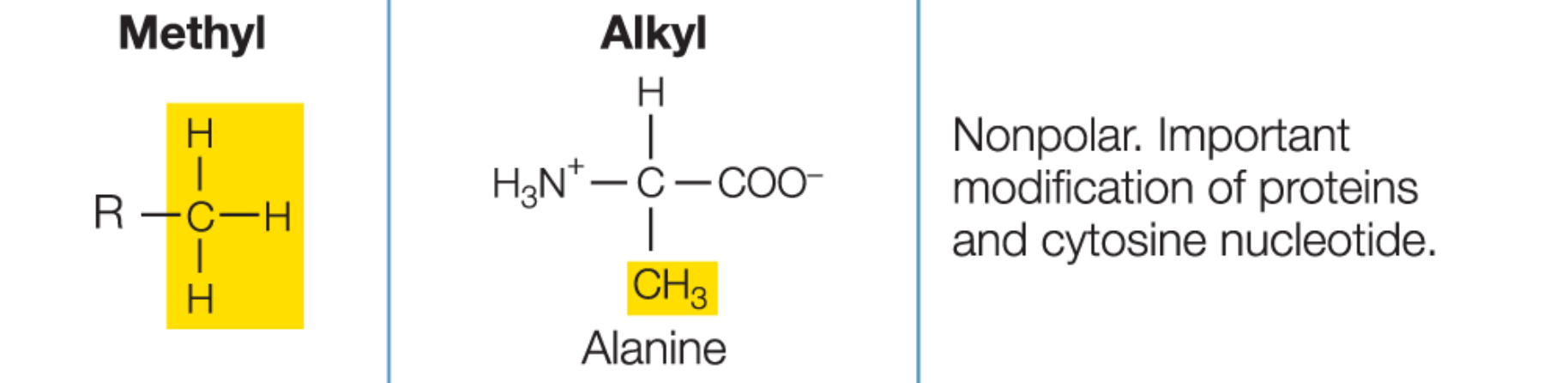
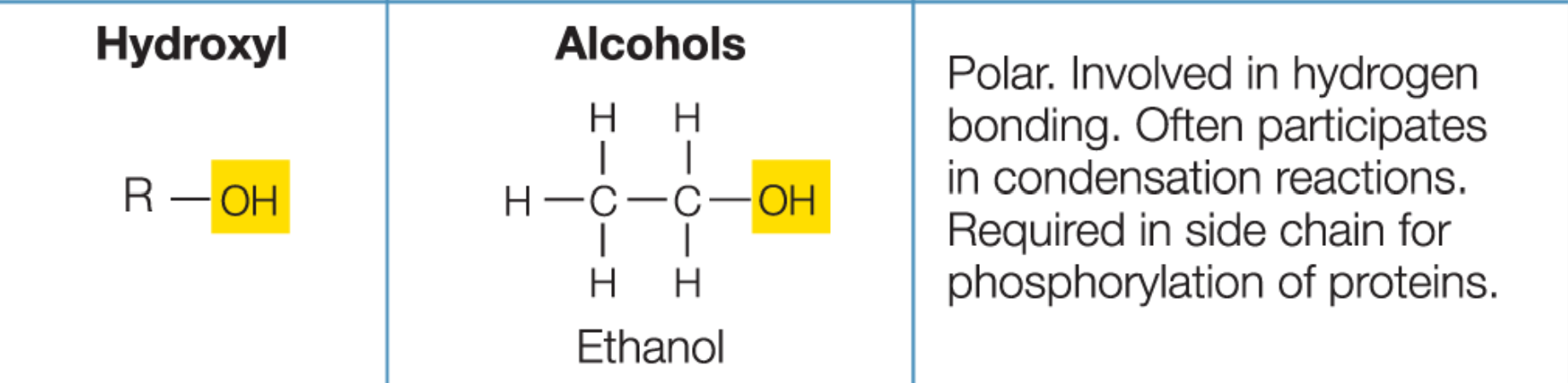
Functional groups
small clusters of atoms that influence the properties and reactivities of molecules
Methyl
Hydroxyl
Sulfhydryl
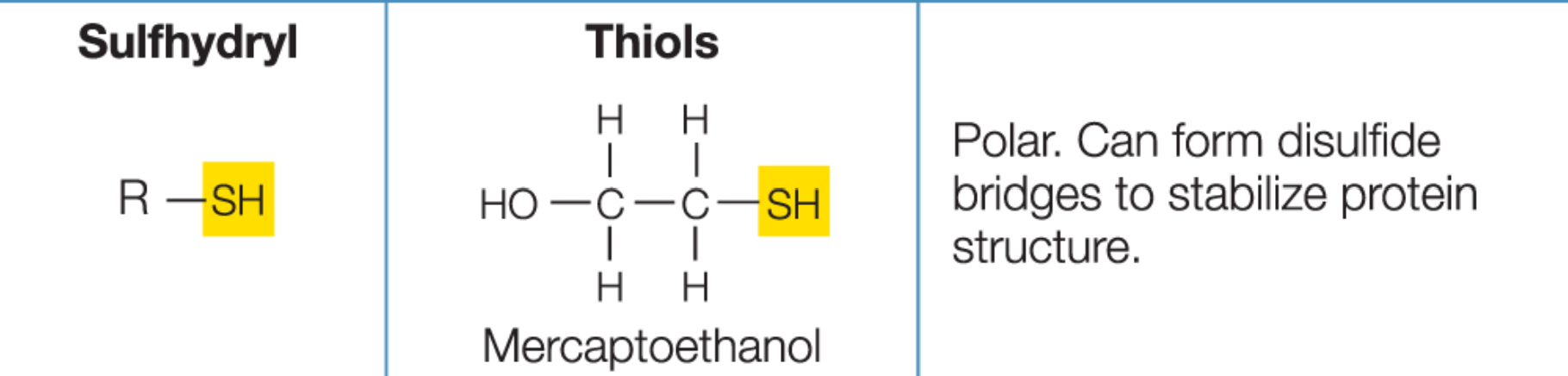
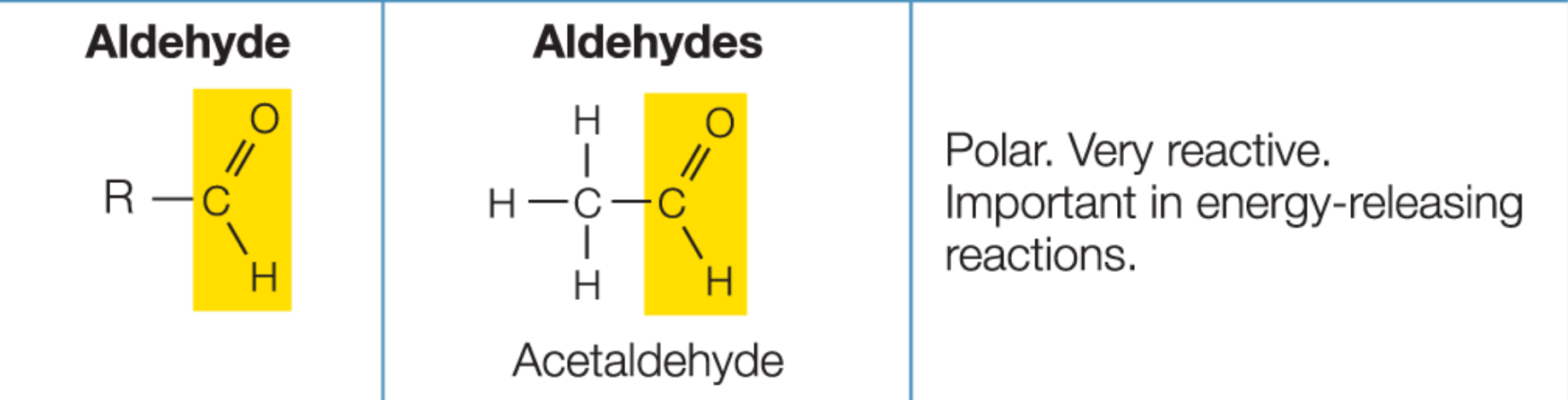
Aldehyde
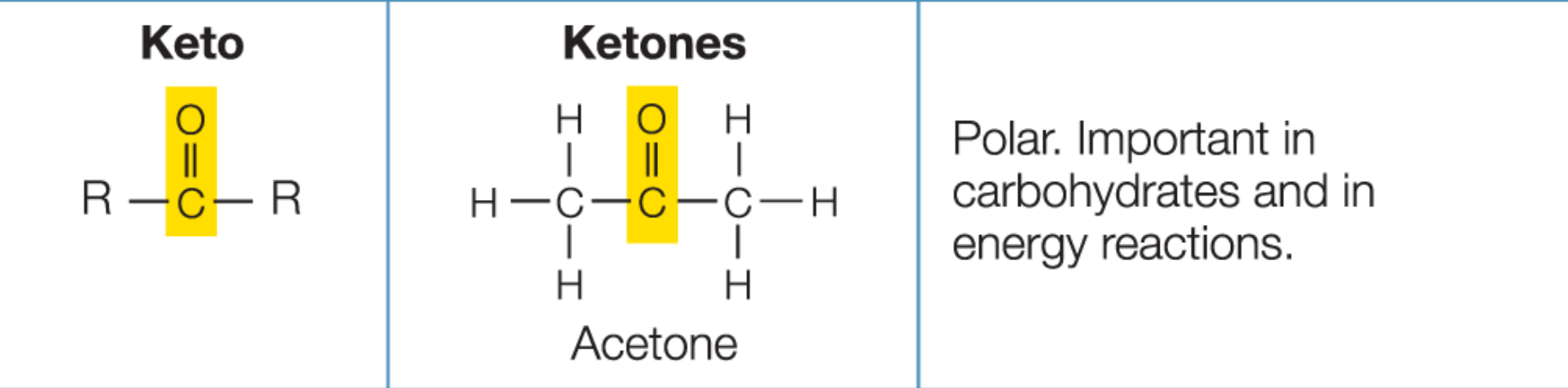
Keto
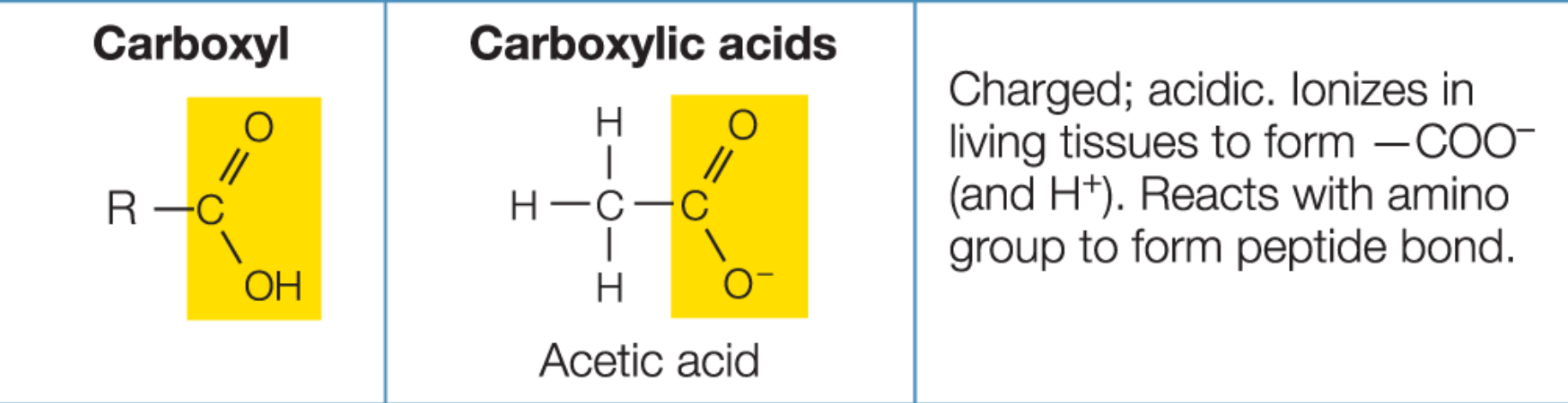
Carboxyl
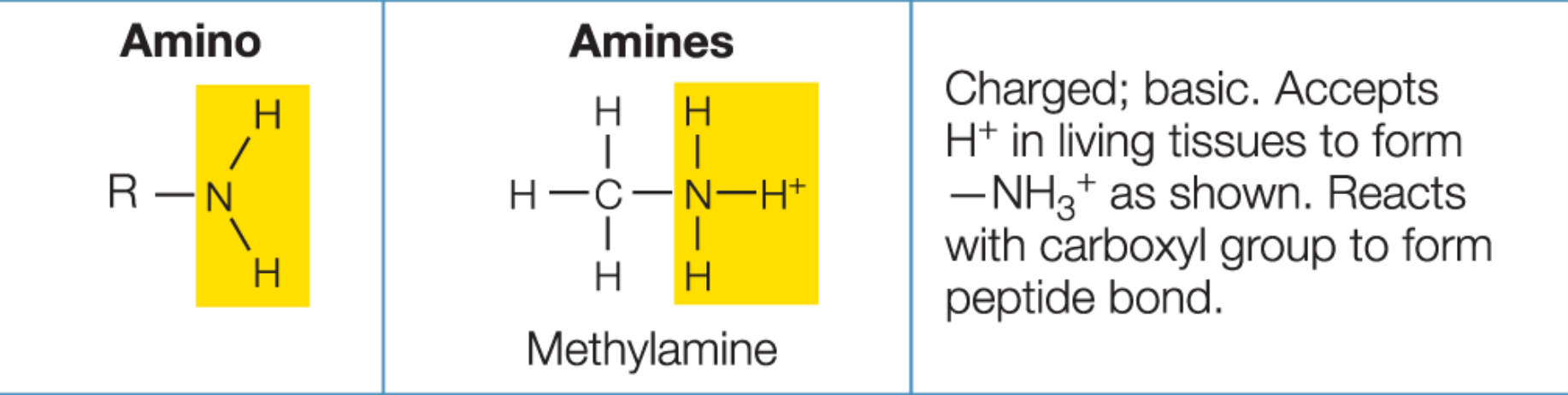
Amino
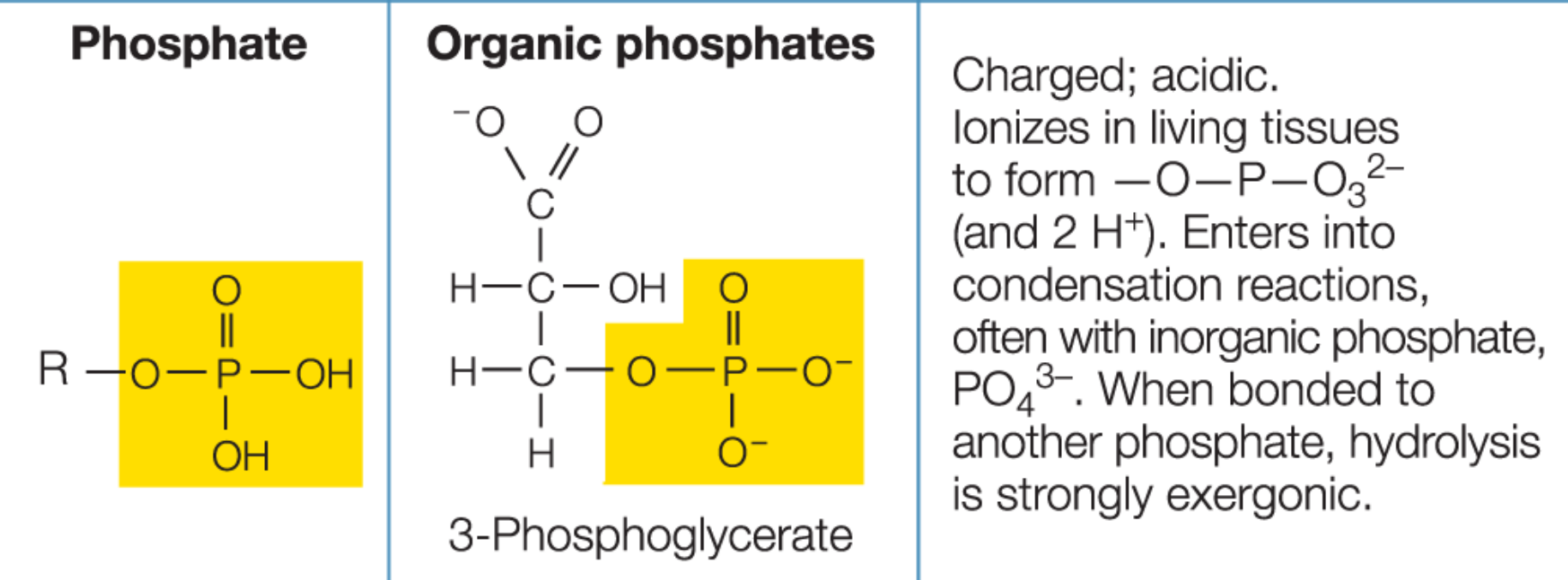
Phosphate
Macromolecules (definition and types)
large molecules formed by covalent bonds between smaller molecules
Carbohydrates, lipids, nucleic acids, proteins
Polymers (definition and types)
Large molecules formed by covalent bonds between smaller molecules called monomers
Carbohydrates, nucleic acids, and proteins are polymers, but lipids are not
Monomers
building blocks of large macromolecules, also called residues
How are polymers created and broken down
Created by condensation reaction, broken down by hydrolysis (reactions involving water)
Lipids (definition, composition, function)
structurally and functionally diverse group of compounds defined by their insolubility in water
Made of hydrocarbons
Hydrophobic because they have nonpolar covalent bonds (C-H and C-C)
Store energy in C-C and C-H bonds
Plays a structural role in cell membranes
Important property of lipids
melting temperature
Melting point is determined by their size and how closely the molecules pack together
Larger lipid molecules and molecules that can pack tightly together have higher melting temperatures because there are more Van der Waals interactions
Held together by van der Waals interactions and the hydrophobic effect (when in water)
White fat
serves to store energy and provide thermal insulation that helps regulate body temperature
Brown fat
gets its color from iron-rich mitochondria and plays an important role in thermoregulation, particularly in infants
Triglycerides
(simple lipids): contains 3 fatty acid molecules and one glycerol molecule, are hydrophobic and nonpolar
Fatty acid
consists of a long nonpolar hydrocarbon chain with a terminal polar carboxyl group (COOH)
Glycerol
3-carbon molecule with 3 hydroxyl groups (OH)
Fatty acids and glycerols are combined by
3 condensation reactions → connected by ester bonds
Ester bond O-C=O
Triglycerides are excellent stores for
chemical-bond energy
2 classifications of triglycerides
Fats: solid at room temperature
Oils: liquid at room temperature
Fatty acid chains can vary in…
length and structure
Composition of fatty acid chains
amphipathic: have a hydrophilic end (COOH) and long hydrophobic tail (many hydrocarbons connected to eachother)
Saturated fatty acids
all the bonds between the carbon atoms in hydrocarbon chain and single bonds because carbons are saturated with hydrogen atoms
Relatively straight molecules and therefore are able to pack closely together, creating a higher melting point
Usually solid at room temperature
Unsaturated fatty acids
hydrocarbon chain has one or more double bonds between carbons atoms because carbons are not fully saturated w/ hydrogen
The double bonds create kinks that prevent unsaturated molecules from packing together tightly → lower melting temperature and are usually liquid at room temperature
Phospholipids
similar to fatty acids but a charged phosphate molecule replaces one of the fatty acids
Also amphipathic
Creates a phospholipid bilayer in water, which is the substance of cell membranes
Lipoproteins
Phospholipids can form single-layer spherical structure that have hydrophobic interiors and hydrophilic exteriors which are used for transporting lipids in aqueous solutions
Carotenoids
lipids that can absorb energy from particular wavelengths of light
Steroids
important lipids in plants and animals, Examples: Cholesterol, Estrogen
Biological roles of carbohydrates (4)
Source of stored energy
Transport stored energy within complex organisms
Structural molecules that give many organisms their shape
Recognition or signaling molecules that can trigger specific biological responses
Carbohydrates
large group of molecules that have similar composition, but differ in several important properties
General formula of carbohydrates
Cm(H2O)n
Simple sugars
carbohydrates with 12 or fewer carbons
Most carbons in carbohydrates are attached to…
hydroxyl groups
Monosaccharides
Simple carbohydrates
Usually in a ring form (sometimes linear)
Consist of 5 or 6 carbons atoms (either called pentoses or hexoses)
3D structures of polymers depends on the isomers of the simple sugars
Different monosaccharides often have the same chemical formula and are structural or stereoisomers
Disaccharides
composed of two monosaccharides joined in a condensation reaction
Glycosidic linkage
bond that connects monosaccharides
The main carbohydrates in cells and readily break down to release energy
Monosaccharides and disaccharides
Oligosaccharides
carbohydrates composed of 3-10 monosaccharides joined by glycosidic bonds
Also can have functional groups which give them additional properties
Often covalently bonded to proteins or lipids, where they serve as recognition signals affecting the molecule’s function and solubility
Polysaccharides
large polymers of hundreds to thousands of monosaccharides connected by glycosidic bonds
2 forms of polysaccharides
Linear chains of monomers (attached via 1,4 glycosidic bonds)
Branched chains of monomers (from 1,6 glycosidic bonds)
Linear polysaccharides
Linear chains can align closely and form hydrogen bonds w/ neighboring chains → form dense sheets or strong fibers that are resistant to breaking
Ex. cellulose: component of plant cell walls (most abundant carbon compound on the planet)
Ex. chitin: arthropod skeletons
Branched polysaccharides
Ex. Starches: principal energy storage compound of plants
Ex. Glycogen: principal energy storage in animals, fungi, and bacteria
Both water-insoluble
Note: starches and glycogen contain both types of bonds
Energy is stored as glycogen instead of glucose because high glucose levels cause water to enter cells
Nucleic acids
polymers that store, transmit, and express genetic (hereditary) information
2 types of nucleic acids
DNA (deoxyribonucleic acid)
RNA (ribonucleic acids)
Nucleotide
building blocks (monomers) of nucleic acids
3 components of nucleotides
Monosaccharide (pentose ribose or deoxyribose)
Nitrogen-containing base
One to three phosphate groups
Nucleoside vs. nucleotide
Nucleosides have no phosphate
Nucleotide is a nucleoside plus 1-3 phosphate groups
Types of bases in nucleic acids
Pyrimidine: single-ring structure
Purine: fused double-ring structure
Purines
Pure as gold (adenine and guanine)
Pyrimidines
Cytosine, Thymine, Uracil
Major differences between DNA and RNA monomers and strands
The monosaccharide in DNA is deoxyribose, while in RNA it is ribose
DNA has nucleotides CTAG (cytosine, thymine, adenine, guanine), while RNA replaces thymine with uracil
RNA is usually single stranded, while DNA is double-stranded