Interpreting and explaining results: effect of pH
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
At Point A of the graph, what happens at the optimum pH for an enzyme-catalysed reaction?
The shape of the active site enables bonds to form successfully with the substrate, resulting in the greatest frequency of enzyme-substrate complex formation and the highest rate of reaction.
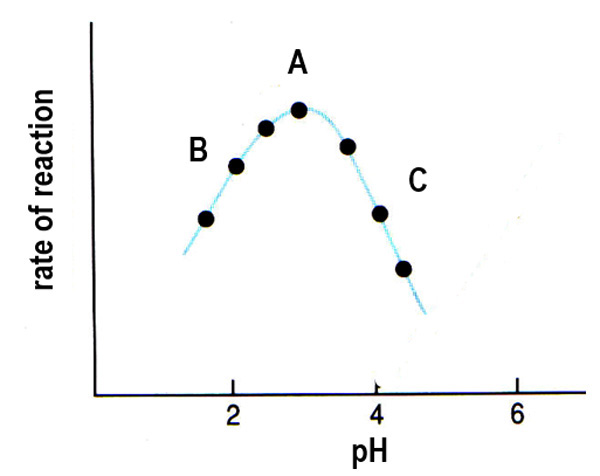
At Point B of the graph, how does low pH affect enzyme activity?
In low pH, a high concentration of H+ ions leads to more amino groups being positively charged, affecting hydrogen and ionic bonding, changing the 3D shape of the active site, and causing a decrease in enzyme-substrate complex formation and reaction rate.
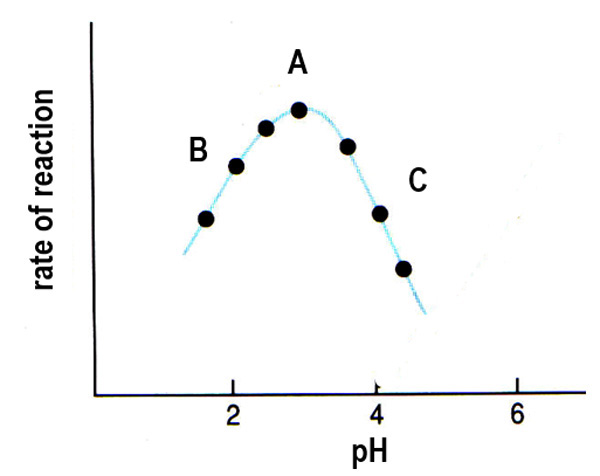
At Point C, what occurs at pH values above the optimum for enzyme activity?
Above the optimum pH, not enough H+ ions are present, leading to more negatively charged carboxylic acid groups, affecting bonding and changing the active site's shape, which decreases enzyme-substrate complex formation and rate.
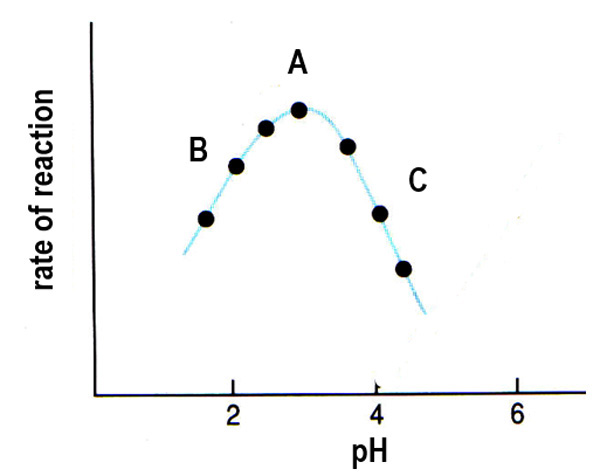
What is the effect of small and large changes in pH on an enzyme's 3D shape?
Small changes in pH are reversible but large changes in pH are irreversible and can permanently change the 3D shape of the polypeptide chain due to the formation of new bonds.
Why is a buffer used when investigating the effect of pH on an enzyme?
A buffer is used to maintain a constant pH because enzymes are sensitive to pH changes.