CH4710 RM22
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
turnover number (kcat)
the number of substrate molecules converted to product in a given unit of time on a single enzyme molecule when the enzyme is saturated
simpler def: how many substrate molecules are transformed into products per unit time
What is the equation for the catalytic efficiency of an enzyme?
kcat/Km (units of M-1s-1)
Interpretation of kcat/Km value
kcat/Km indicates how effective the enzyme is on a particular substrate
as ratio increases, rate of catalysis increases
diffusion-controlled reactions
reaction rate = the rate of transport of the reactants through the solution/medium where they are
2 classes of enzyme inhibitors
reversible
irreversible
4 types of reversible inhibition
competitive
uncompetitive
mixed
noncompetitive
competitive inhibitor
competes with the substrate for the active site of an enzyme
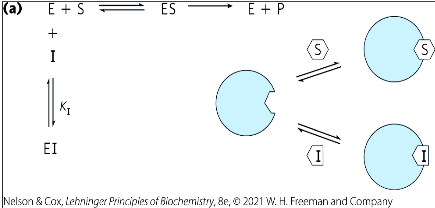
uncompetitive inhibitor
binds at a site that’s not the active site
binds only to the ES complex
What is the difference between competitive and uncompetitive inhibition?
In competitive inhibition, an inhibitor will bind to the enzyme’s active site so that the substrate can’t.
In uncompetitive inhibition, the substrate will bind to the enzyme’s active site, forming the ES complex. Then, an inhibitor will bind to a separate site.
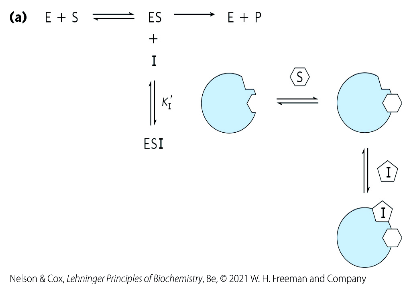
non-competitive inhibitor
binds to site that’s not an active site
has equal affinity to E and ES forms
What is the difference between non-competitive and uncompetitive inhibition?
Non-competitive inhibition has equal affinity to E and ES forms.
Uncompetitive inhibition will only have the ES form.
KI equation
KI = [E][I]/[EI]
([I] is the concentration of inhibitor)
Values of alpha (⍺) equation
⍺ = 1+ [I]/KI
How does ⍺ conclude where an inhibitor will bind/what type of inhibition will be carried out?
⍺ > 1.0 means binding is on the enzyme E (competitive)
⍺ < 1.0 means binding is on the ES complex (uncompetitive)
⍺ = 1.0 means inhibitor has equal affinity to E and ES forms (non-competitive)
irreversible inhibition
the irreversible inhibitor will bind covalently with or destroy a functional group on an enzyme that is essential for the enzyme’s activity
OR the irreversible inhibitor can also form a highly stable noncovalent association with the functional group
2 types of irreversible inhibitors
suicide substrates (SS)
transition-state analogs
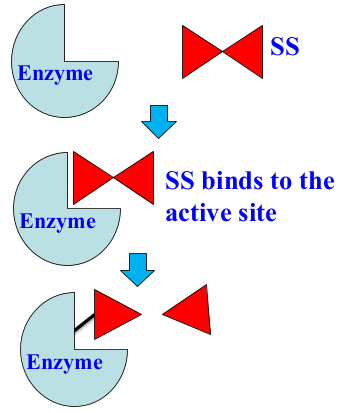
Describe the process of suicide substrate inhibition.
the SS binds to the active site of the enzyme
the SS is converted to a product
a part of the product forms a covalent bond with the enzyme, while the other part leaves
enzyme becomes inactive
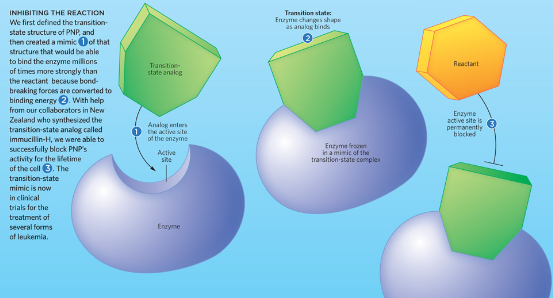
transition-state analogs
stable molecules designed to resemble transition states
bind to enzyme active site more tightly than the substrate can
blocks the substrate permanently from active site
ex. HIV Protease inhibitor (HIV protease makes small viral proteins that form viruses)
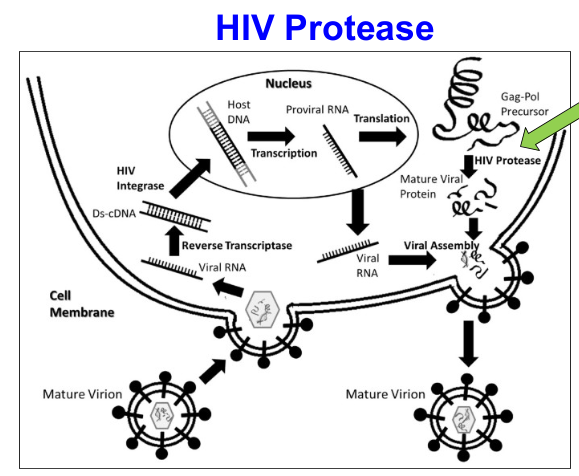
Describe/explain
HIV Protease cleaves the large Gag-Pol precursor protein to make smaller viral proteins, which are important for the survival of the virus.
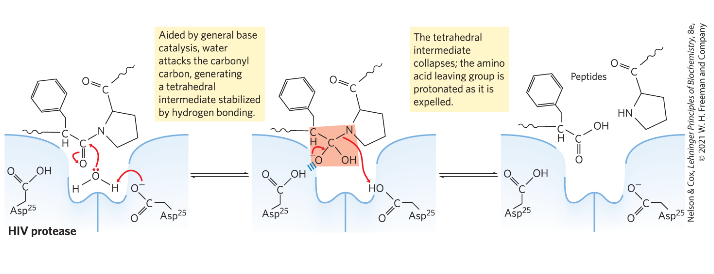
Describe/explain
mechanism action of HIV protease
HIV protease is dimer
water attacks carbonyl carbon
tetrahedral intermediate is formed
intermediate collapses
amino acid leaving group is expelled