biochem ii 2.5 amino acid catabolism
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
in general, amino acids are catabolized to two classes of metabolites. name + describe each
ketogenic, which are converted to metabolites that can undergo ketone body synthesis or fatty acid biosynthesis
glucogenic, which are converted to metabolites that can enter gluconeogensis
**several amino acids can do both, such as threonine!
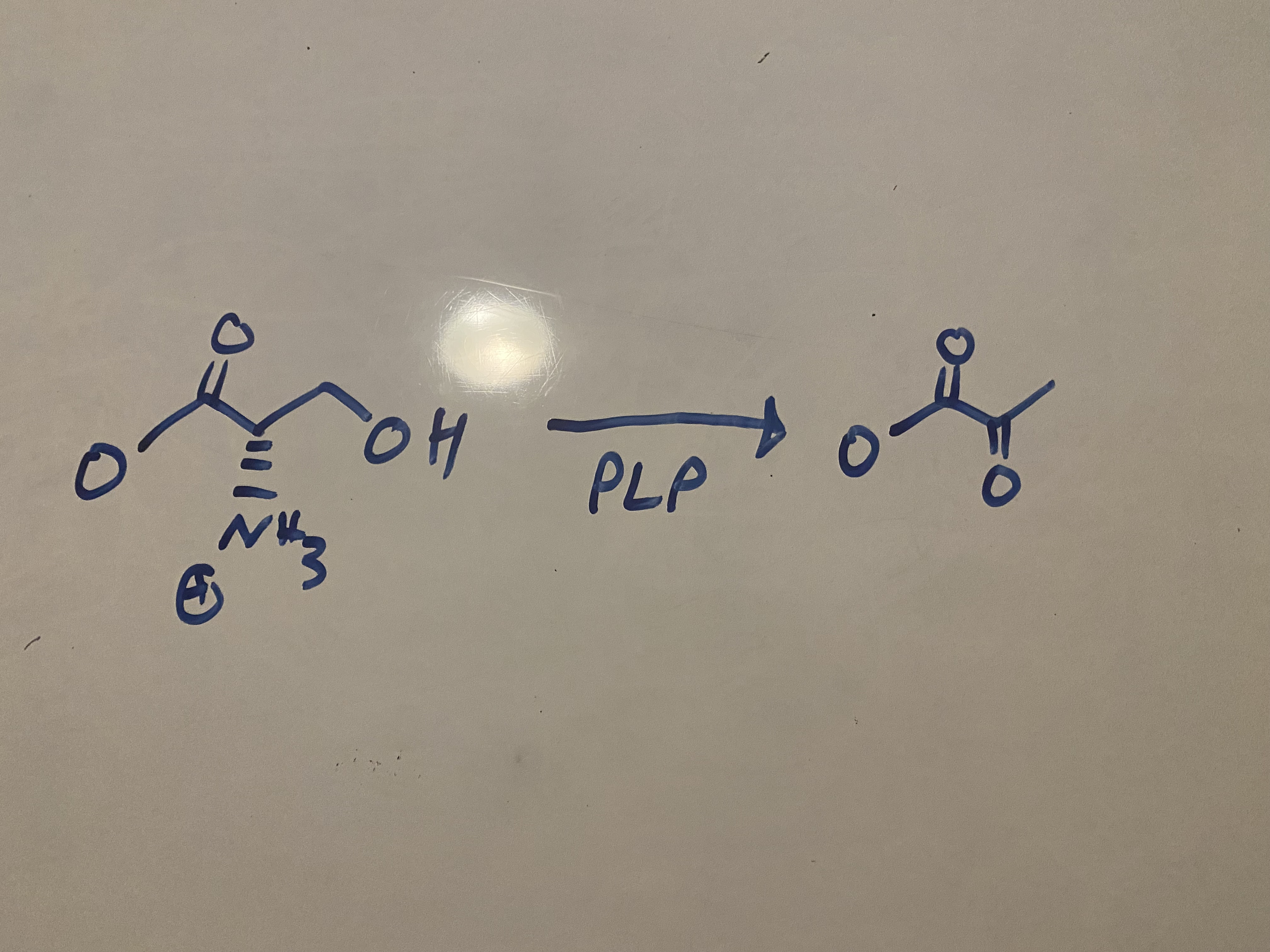
what catabolic rxn does serine undergo?
conversion of serine to pyruvate using PLP (pyridoxal phosphate)
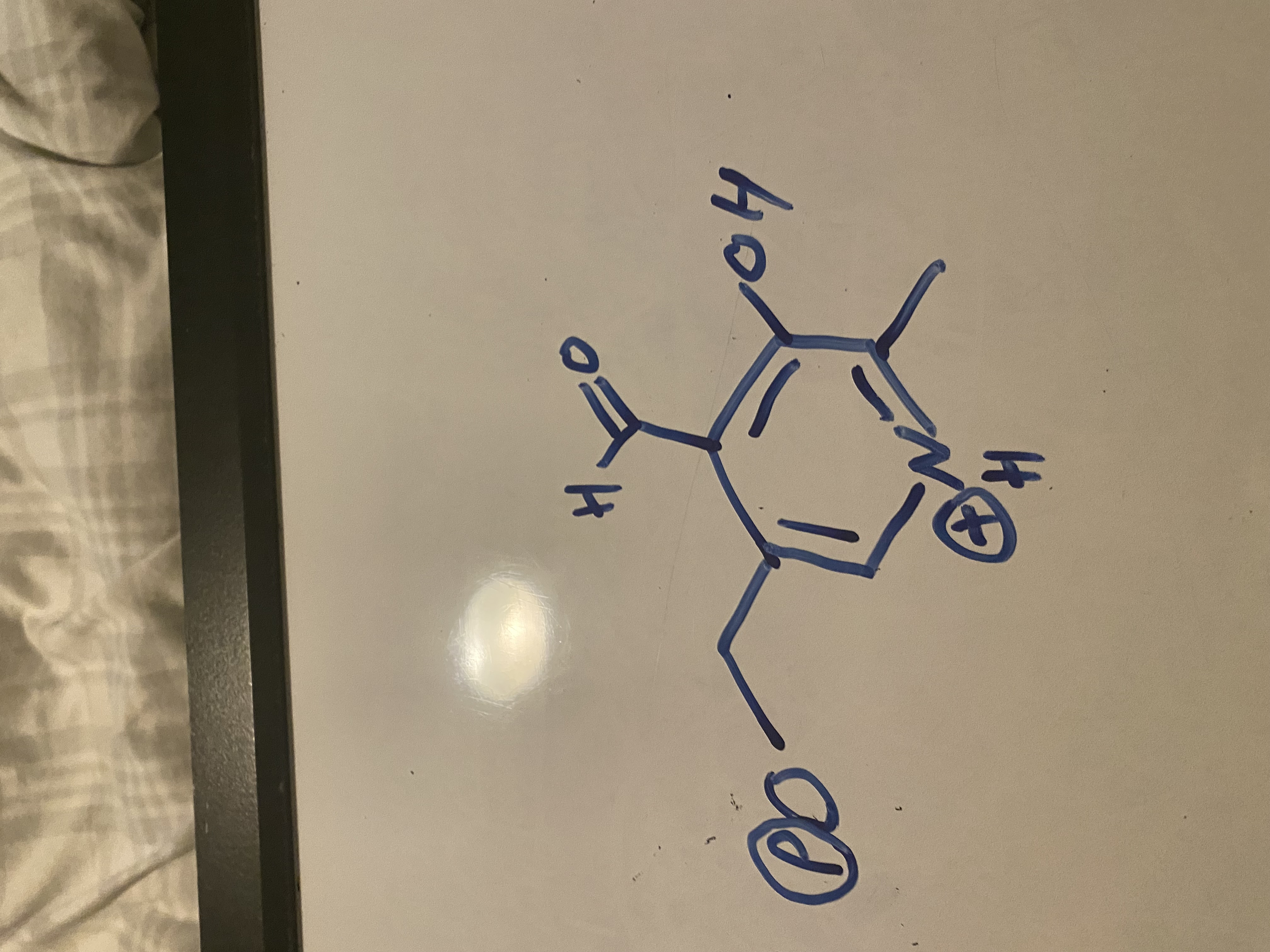
draw PLP structure
draw mechanism for conversion of serine to pyruvate using PLP (pyridoxal phosphate) — abbreviate PLP structure
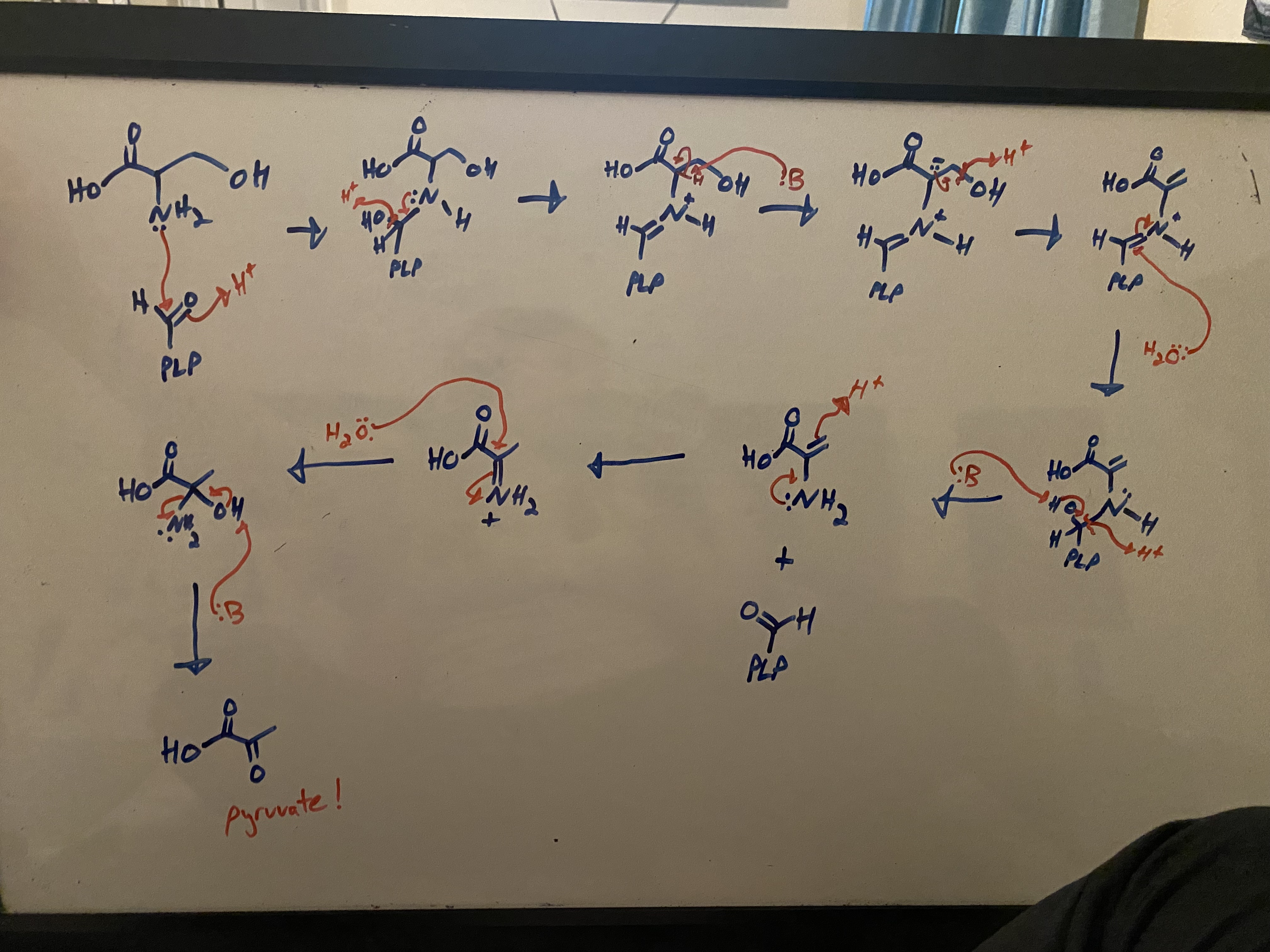
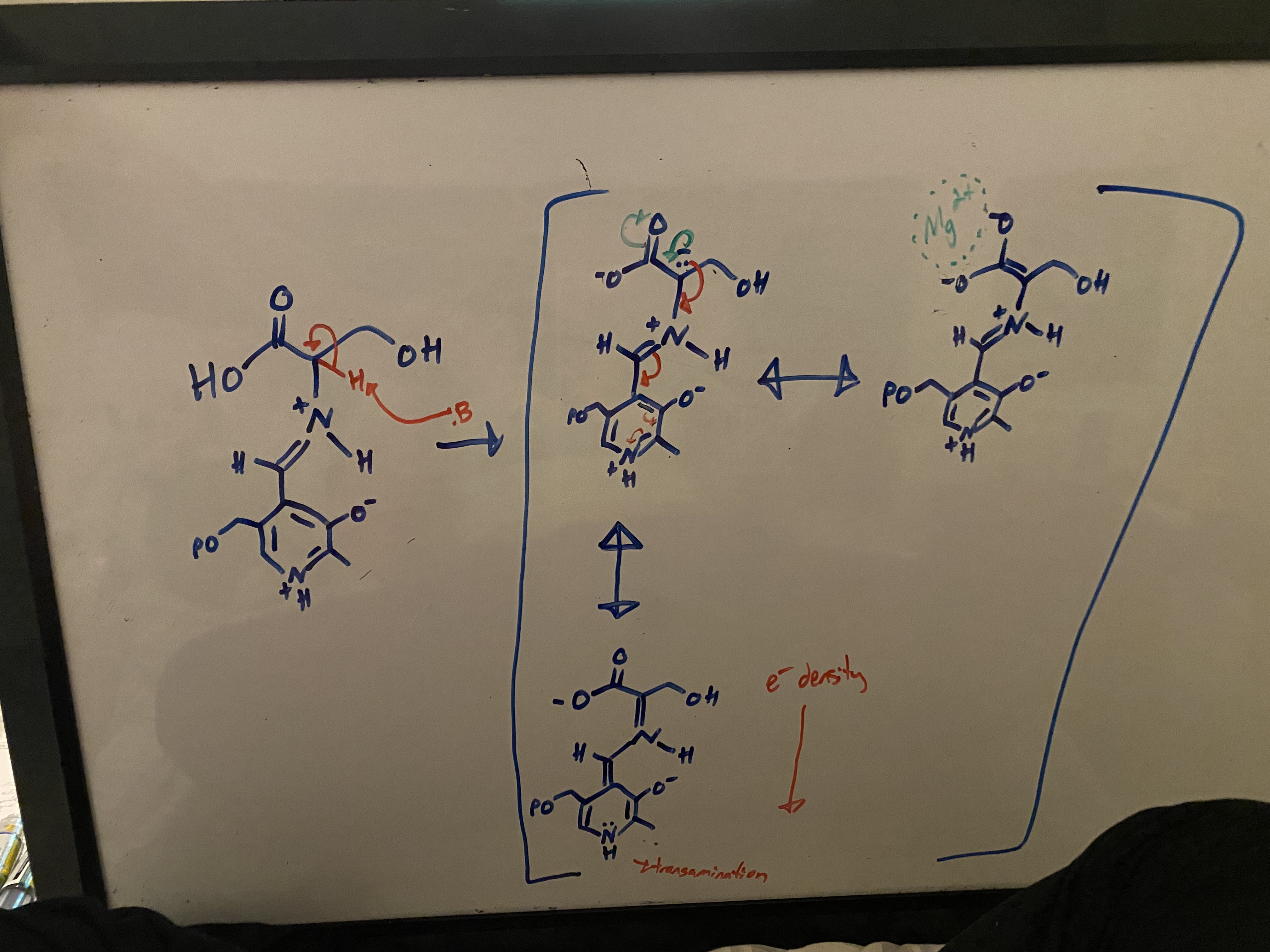
step 3 of the conversion of serine to pyruvate using PLP (pyridoxal phosphate) involves the loss of an acidic hydrogen. prove that this hydrogen is acidic via resonance structures
high degree of resonance stabilizes the conjugate base — this is the DEFINITION of what determines the strength of an acid!
what catabolic rxn does asparagine undergo?
conversion of asparagine into an aspartate intermediate, which is then transaminated to produce oxaloacetate
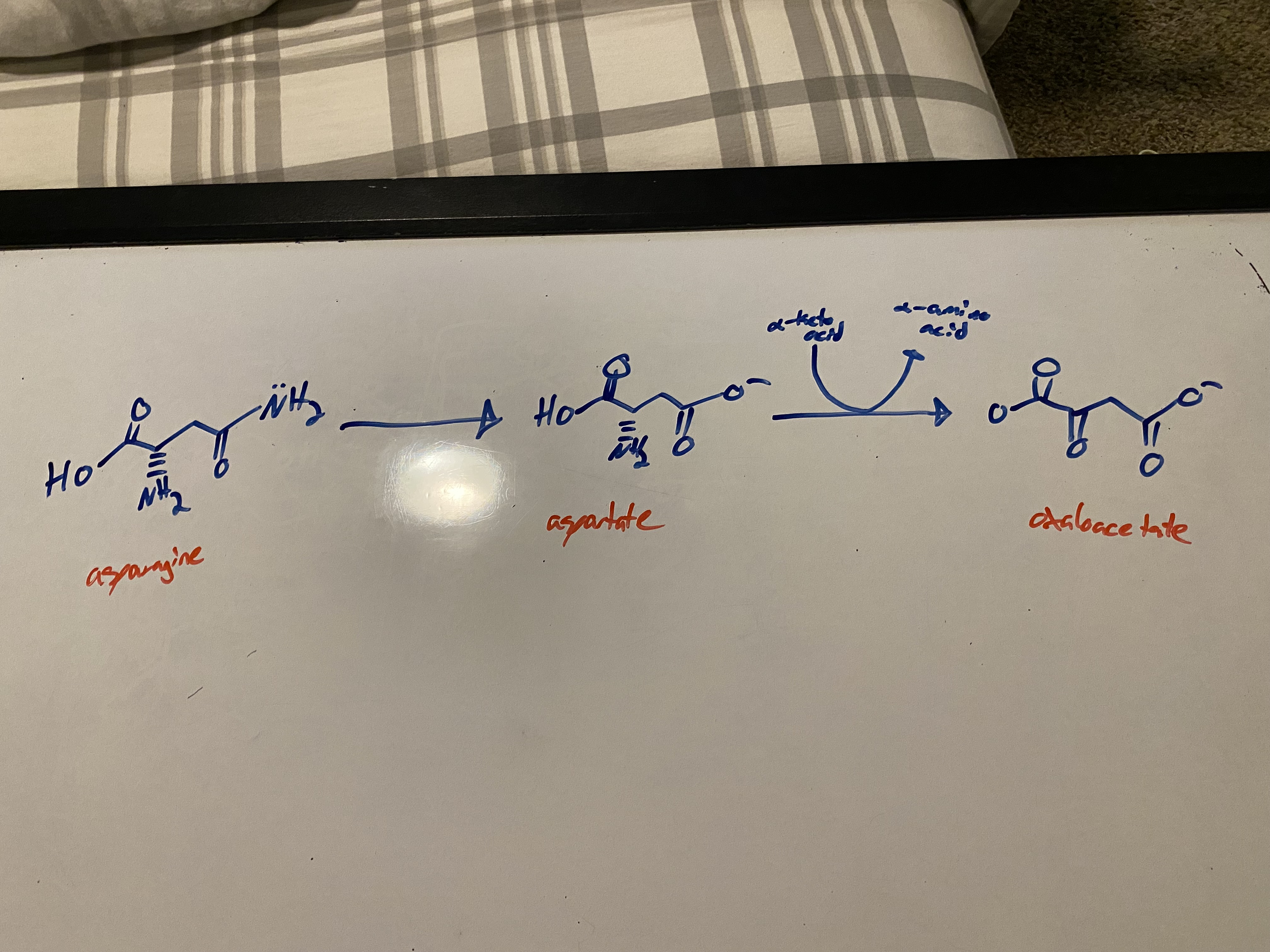
mechanism for conversion of asparagine into an aspartate intermediate, which is then transaminated to produce oxaloacetate
**this rxn only uses WATER!
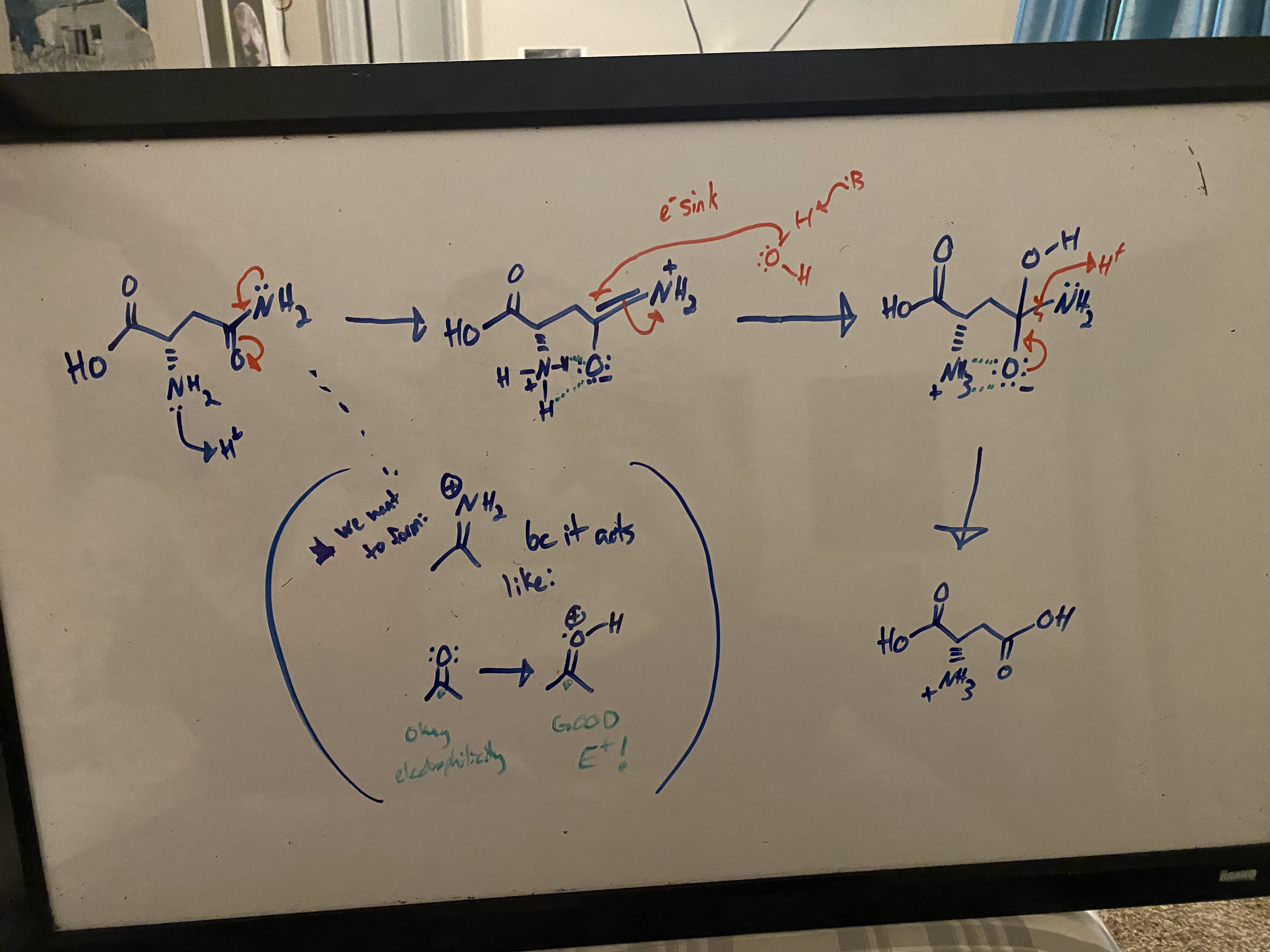
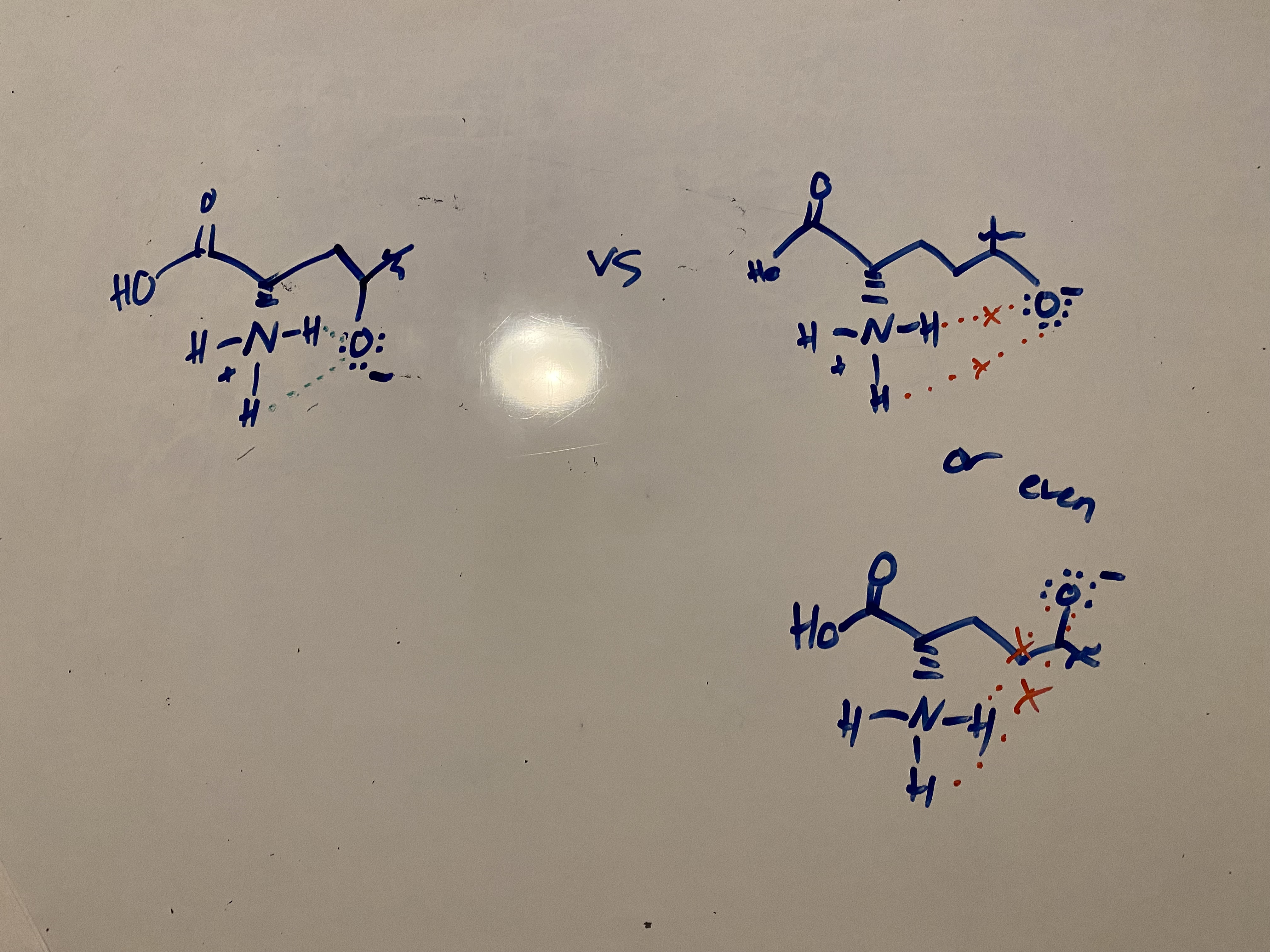
consider the following… for the sake of this brainteaser, let’s say that glutamate undergoes a very similar process as asparagine (which it may or may not actually do irl). supposing the mechanism is essentially identical, explain why this reaction is MUCH SLOWER for glutamine catabolism compared to asparagine catabolism
in asparagine catabolism, the interaction between the NH3+ and the O- provides stability to the molecules and their transition states, thus speeding up the reaction substantially.
glutamine catabolism is slower because these molecules are too far apart and cannot benefit from this stabilizing interaction