Biochem Exam 2 to End
1/86
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
87 Terms
What are the 3 classes of lipids and what do they commonly do
Free fatty acids which are a common fuel, triacylglycerol which are a storage form, and membrane lipids which are phospholipids (membranes), glycolipids (membrane lipids w carbs), and steroids (polycyclic hydrocarbons w a variety of functions)
What is the definition of fatty acid
Hydrogen bearing carbon chain with a carboxylic acid at one end and methyl on the other
Saturated vs unsaturated fatty acid
Unsaturated has double bond, saturated is made of all single bonds
How is nomenclature of fatty acids achieved (numbering, alpha beta, double bond, and naming)
The numbering starts at the carbon of the carboxylic group. Atom 2 is the alpha carbon and atom 3 is the beta carbon. Triangle indicates double bond. Ionized is usually the name as this is the physiological form.
Fatty acids even/odd and what config are they
Usually there is an even number of carbon atoms w double bonds, usually cis config
What are polyunsaturated fatty acids separated by
At least one methylene group
How do double bonds and chain length affect polyunsaturated fatty acids
Cis double bonds increase fluidity as well as short chain length
Why are cis polyunsaturated fatty acids important
They are precursors to hormones and therefore essential in our diet
What are triacylglycerol composed of
Three fatty acids and glycerol backbone
Where are triacyclglycerols housed in the body and why do they hold more energy than glycogen
Adipose tissue, and it is because they are anhydrous
What is meant when you say that membrane lipids are amphipathic
The fatty acids are the hydrophobic part and the alcohol/phosphate are hydrophilic
What are phospholipids made of
Fatty acids (2 or more), a platform (glycerol or sphingosine), phosphate, and an alcohol
What is a phospholipid that uses glycerol and a phospholipid that uses sphingosine called
Glycerol is called phosphoglycerates and sphingosine is called sphingolipids
What is the importance of sphingomyelin
Sheath in nerve cells
What are glycolipids and what do they do
Glycolipids contain carbohydrates and play a role in cell/cell recognition on the extracellular surface
What are steroids
Tetracyclic platform with three cyclohexane rings fused with cyclopentane
What is the most common steroid and what does it do
Cholesterol is the most common and it is a precursor to hormones
What are the two ways that glycerol 3-phosphate can be obtained
Dihydroxyacetone phosphate getting turned with NADH and glycerol being phosphorylated
How does glycerol 3-phosphate turned into phospholipid or triacylglycerol
Glycerol 3-phosphate has two fatty acids added to make phosphatide. Then phosphatidate can react with triacylglycerol synthase on the ER to make triacylglycerol or they can react w an alcohol to make a phospholipid
What is the significance of Phosphatidic acid phosphatase
Main regulator enzyme converting between phosphatidate and diacylglycerol (intermediate between phosphatidate and triacylglycerol). These both also function as second messengers
How is cholesterol synthesized
Mevalonate made by HMG CoA reductase which is the committed step then this is turned into isopentyl pyrophosphate, six molecules of isspentyl pyrophosphate is turned into squalene, then squalene cyclized and is turned into cholesterol
Expand on how mevalonate is made then 3-isopentyl pyrophosphate
Three acetyl CoA molecules make the Mevalonate (2 NADPH + 2H → 2NADP+ + CoA). Then Mevalonate gets turned into 3-isopentyl pyrophosphate by three phosphorylations and one decarboxylation
Expand on how six 3-isopentyl is turned into squalene
C5 → C10 → C15 (Farnestyl pyrophosphate) which combines the two molecules to make squalene
Where is the main site of cholesterol synthesis
The liver is the main site where synthesis HMG CoA reductase
What are the three stages of fatty acid degredation
Degradation of the TAG into blood for transport, 2. Activation of the fatty acids and transport into the mitochondria, 3. Degradation to Acetyl CoA for processing (Oxidation, hydration, oxidation, and cleavage by thiolase)
How are triacyclglycerols degraded and into what
Lipases cleave triacyclglycerols into three fatty acids and glycerol
What happens to the glycerol in the triacylglycerol degradation
Glycerol is not wasted, it enters the liver and can be metabolized by glycolytic or gluconeogenic pathways. Can be turned into dihydroxyacetone phosphate which can easily be turned into glyceraldehyde 3 phosphate
How do epinephrine and glucagon play a role in TAG degredation
Glucagon and epinephrine activate a g coupled protein that turns ATP → cAMP, activating protein kinase A, furthermore turns on lipase (phosphorylates) and perilipin which helps mobilize
How does the hormone-mediated degradation of TAG’s get coordinated with metabolism
At the same time as TAG degradation, glycogen gets broken down
How are fatty acids activated and what is special about the reaction
Fatty acids are joined with CoA to make Acyl CoA. This happens by first Acyl adenylate being made by breaking down ATP → AMP, then CoA displacing the AMP making Acyl CoA. This reaction is made irreversible by pyrophosphatase
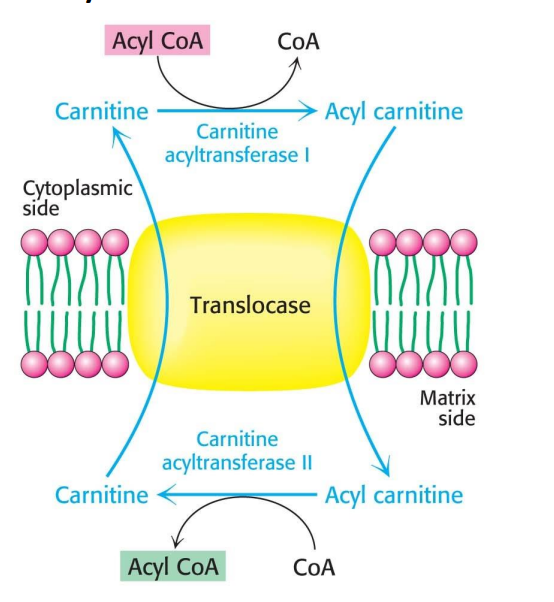
How are fatty acids brought into the mitochondria?
The fatty acid (acyl) gets transferred to carnitine to make acyl carnitine, and the acyl carnitine translocase allows for penetration into the membrane. Acetyl cartnitine transferase is responsible for the transfers of the acyl group
What is an alternate fuel source synthesized by fatty acids and what are they
Ketone bodies which are made from acetyl CoA in the liver mitochondria and secreted into the blood
How are ketone bodies formed?
Two acetyl CoA’s make acetoacetyl CoA which gets another acetyl CoA to make an intermediate which quickly gets turned into acetoacetate which spontaneously turns to acetone and the formation of D-3-hydroxybutyrate by breaking up NADH
Describe diabetic ketosis
No insulin causes fats to be released from adipose tissue. Fats are degredated by the liver but cannot process acetyl CoA. Excess ketone bodies form, lowering pH, causing coma and death.
What happens to fuel sources during starvation
Protein degredation is the initial source of carbs, and after a few days ketone bodies are used as fuel for the brain. The ketones prevent the substantial degredation of tissues.
How does D-3-hydroxybutyrate get used as fuel
D-3-hydroxybutyrate reacts with a dehydrogenase to make acetoacetate (and NADH) which gets turned into acetoacetyl CoA (generating succinate), then 2 Acetyl CoA’s by thiolase.
What are the three stages of synthesis of fatty acids
Transfer of acetyl CoA out of mitochondria to cytoplasm, acetyl CoA activation into malonyl CoA, and then the repetitive addition and reduction of two carbon units to synthesize C16 fatty acid, with synthesis occurring on an acyl carrier protein.
How is Acetyl CoA taken out of the mitochondria
Acetyl CoA is turned into citrate by combining with oxaloacetate, which moves out into the cytoplasm, the acetyl CoA gets removed and this leaves oxaloacetate, then malate is made with NADH, which then turns to pyruvate with the removal of NADPH.
What does fatty acid synthesis use for reducing power and how is it produced
NADPH is used for reducing power and it can be produced by the oxidation of oxaloacetate
How Malonyl CoA is synthesized and what’s the significance
It is the committed step and is done by Acetyl CoA carboxylase.
What are the two steps of malonyl CoA synthesis
The first step is biotin-enzyme complex using ATP to make CO2 Biotin complex, then the CO2 Biotin works on acetyl CoA to make malonyl CoA and revert biotin-enzyme EXAMPLE OF DOUBLE DISPLACEMENT
What is the enzyme that catalyzes the formation of fatty acids and how does it work
Fatty acid synthase catalyzes the formation on acyl carrier proteins, making Acyl ACP and Malonyl ACP. It then does the condensation, reduction, dehydration,and reduction reactions.
Expand on the condensation, reduction, dehydration, and final reduction steps of fatty acid synthesis
Acetyl ACP + Malonyl ACP combine to make Acetoacyl ACP, then this is reduced w NADPH, then a dehydration, and another reduction using NADPH. This is repeated until palmitate is made.
How does the fatty acid synthesis terminate
The cycle will be continued until a C16 body is formed. The ACP is cut off at the end, leaving palmitate
What is the structure of the enzymes that work in fatty acid synthesis
Animals have entire enzyme on a single polypeptide chain. They are two identical chains (a homodimer). There are two distinct components with one selecting and condensing, and the other modifying part which reduction/dehydration
Where are longer fatty acids synthesized and how does this work
They are made by enzymes on the endoplasmic reticulum. These enzymes extend palmitate by adding two c units, using malonyl CoA.
How are double bonds introduced into saturated fatty acids and how does this play into essential fatty acids
Enzymes bound to the ER add the double bonds. Mammals lack the agbility to add double bonds past carbon 9, making essential fatty acids
How is Acetyl CoA carboxylase regulated
1. Carboxylase 1, a cytoplasmic enzyme, is inhibited when phosphorylated by AMP-activated kinase (AMPK). Inhibition due to phosphorylation is reversed by protein phosphatase 2A. 2. Citrate actives carboxylase by facilitating the formation of active polymers of the carboxylase. Citrate mitigates inhibition due to phosphorylation. 3. Palmitoyl CoA, the end-product of fatty acid synthase, inhibits carboxylase by causing depolymerization of the enzyme. 4. Acetyl CoA carboxylase is regulated by a variety of hormones
How specifically does citrate work to promote active carboxylase
Inactive dimers get combined with the help of citrate to make active filaments, palmitoyl CoA inhibits carboxylase by depolymerization,
How is Acetyl CoA carboxylase regulated by hormones
Glucagon and epinephrine enhance AMPK activity (AMP activated kinase). Insulin stimulates the dephosphorylation of carboxylase.
How are amino acids turned into ammonium ions
Amino groups are transferred onto alpha ketoglutarate by aminotransferases to make glutamate which then is deaminated to make NH4
What enzyme is responsible for the deamination and what does it make
Glutamate dehydrogenase releases the NH4 (mitochondrial) also producing NADH or NADPH
Which amino acids can be directly deaminated
Serine makes pyruvate + NH4 while Threonine makes alpha ketobutyrate and NH4
What happens to the NH4 and where does this occur in humans
The excess ammonia ions are ultimately converted to urea in the urea cycle. This occurs within the liver of humans
What is the committing step of the urea cycle
This is the first step where ammonia is coupled with bicarbonate to make carbamoyl phosphate, and is facilitated by carbamoyl phosphate synthetase (CPS I)
What happens to carbamoyl phosphate once it is formed
Carbamoyl phosphate combines with ornithine to make citrulline, which is transported out of the mitochondria
What happens to citrulline after it is brought into the cytoplasm and then the next step followed by restarting the cycle
Citrulline is combined with aspartate to make arginosuccinate. Arginosuccinate is cleaved into fumarate and arginine. The arginine is cleaved into urea which is excreted and ornithine is transported back into the mitochondria to restart the cycle
How is the urea cycle, citric acid cycle, and transamination of oxaloacetate linked and how
Fumarate and aspartate. Fumarate can be converted into oxaloacetate by the citric acid cycle and then glucose by the gluconeogenic pathway
What happens with defects in the liver in terms of urea synthesis
Defects in any of the urea cycle enzymes increase NH4 in the blood, which is fatal
What is a ureotelic organism vs ammoniotelic organism vs uricotelic
Ureotelic organisms excrete nitrogen as urea while ammoniotelic organisms excrete directly as NH4. Uricotelic organisms like birds excrete uric acid.
What is phenylketonuria
A lack of phenylalanine hydroxylase, and excess phenylalanine is turned into phenylpyruvate, leading to impaired mental ability.
Whats the difference between plants/bacteria and animals in terms of amino acid synthesis
Plants and bacteria make all amino acids themselves while animals need to get some from their diet
Where do the carbon skeletons of amino acids come from
Intermediates of pentose phosphate pathway, glycolytic pathway, and the citric acid cycle
What are the two types of bacteria that can fix nitrogen (and third natural way)
Free-living bacteria which are non symbiotic and mutualistic bacteria which are symbiotic. Lightning can also fix nitrogen.
What enzyme is responsible for nitrogen fixation and how does it work
Nitrogenase enzyme complex is responsible. There are two parts, the reductase provides high energy electrons (ferredoxin) for reducing power while the nitrogenase uses the electrons to reduce N2 into NH3
What is the industrial process that fixes nitrogen
The Haber Process
Why does nitrogenase need ATP
N2 reduction is thermodynamically favorable but breaking the double bonds requires a ton of energy.
What is gluconeogenesis and where does it occur
Gluconeogenesis is the synthesis of glucose from non-carbohydrate precursors occurring primarily in the liver and some in the kidney
Which enzymes are not located in the cytoplasm for gluconeogenesis
All enzymes are located in the cytoplasm other than pyruvate carboxylase (mitochondria) and glucose 6-phosphaase (bound to the ER)
What are the three irreversible steps that have to be bypassed
Glucose + ATP to Glucose 6 phosphate, Fructose 6 phosphate + ATP to fructose 1,6 bisphosphate, and phosphoenolpyruvate + ADP to pyruvate
How does pyruvate get turned into phosphoenolpyruvate
Uses two enzymes; pyruvate carboxylase which is in the mitochondria, turning the pyruvate into oxaloacetate (which is tuned into malate to leave the mitochondria then back) as well as phosphoenolpyruvate carboxykinase which turns the oxaloacetate to phosphoenolpyruvate (using GTP)
How does fructose 1,6 bisphosphate get turned into fructose 6 phosphate
This reaction is catalyzed by fructose 1,6 bisphosphatase
How is glucose 6 phosphate turned into glucose in the final step
Glucose 6 phosphate is transported into the lumen of the ER with glucose 6 phosphatase catalyzing the reaction. G6P is transported in with one transporter, with two more transporters for pi and glucose respectively.
What determines if gluconeogenesis or glycolysis prevails?
Energy charge. ATP needed? then glycolysis. Glucose needed? then gluconeogenesis
What is the significance of fructose 2,6 bisphosphate
Stimulates PFK and inhibits Fructose 1,6 bisphosphatase
What is interesting about the enzyme that makes and hydrolyzes fructose 2,6 bisphosphate
The PFK (synthesis) and fructose bisphosphatase (hydrolyzes) are on the same polypeptide chain meaning that it is a bifunctional enzyme
What kind of regulation occurs in the interconversion between fructose 1,6 bisphosphate and fructose 6 phosphate (glucagon F 2,6 BP)
Glucagon stimulates PKA, activating FBPase which increases gluconeogenesis and helps degradation of fructose 2,6 bisphosphate. High Fructose 6-phosphate levels stimulate phosphoprotein phosphatase which removes the phosphate on PFK 2 which makes fructose 2,6 bisphosphate that simulates PFK
What is the significance of lactate in the muscle in terms of gluconeogenesis
The lactate is brought to the liver where glucose is made and brought back into the muscle for use
How does signal transduction work
Signal gets received, then amplified to transduction, which them leads to response (s)
What is the difference between fast and slow response
Fast response immediately creates an altered protein response while slow response goes though DNA to make a protein product
What are the 5 parts of signal transduction that are in common
1. Release of a primary message as a response to a physiological circumstance. 2. Reception of the primary message by a receptor, often an integral membrane protein. 3. Relay of the detection of the primary message to the cell interior by the generation of a intracellular second message. 4. Activation of effector molecules by the second messenger that result in a physiological response. 5. Termination of the signal cascade!
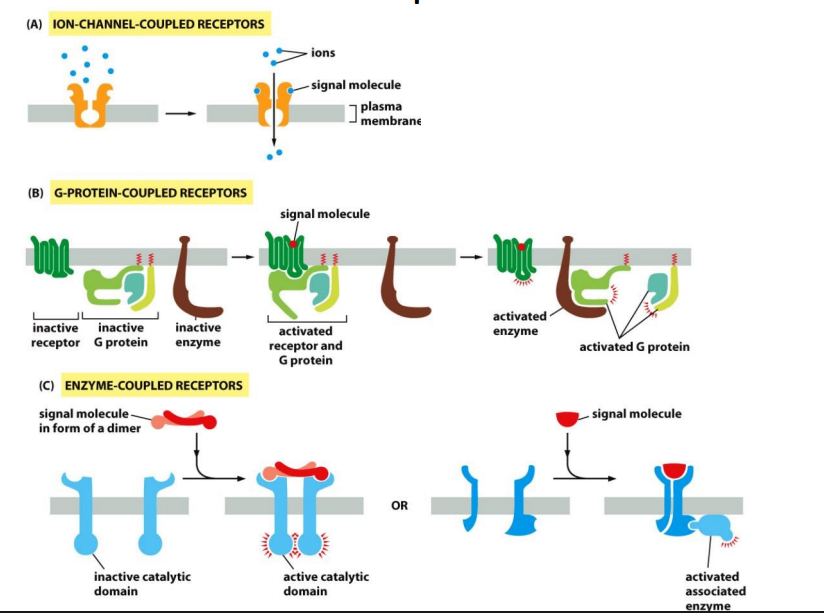
What are the three major classes of surface receptor proteins
Ion-channel-coupled receptors, g protein coupled receptors, and enzyme coupled receptors
What are the ways that extracellular signals can act short or long distances
They can be close and contact dependent w/membrane bound signaling, long distance by synaptic, paracrine with local mediators through signaling cells, and Endocrine through hormones in the bloodstream
What are the six steps in the lifecycle of a hormone
Biosynthesis, secretion of the hormone, transport of the hormone, recognition, relay/amplification, degradation and reset
What is a second messenger and some examples
A non protein molecule in the cell that act to transmit signals from a receptor to a target. Some examples are calcium and cyclic AMP/GMP
How is HMG CoA Reductase regulated
Rate of synthesis is controlled by Sterol Regulatory element binding protein (SREbp) 2. Rate of translation control by mevalonate and cholesterol 3. Phosphorylation of HMG CoA by AMP dependent kinase 4. Proteolytic degradation with increased cholesterol concentration