covalent bonding
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
what is covalent bonding
Non-metal atoms can share electrons with other non-metal atoms to obtain a full outer shell of electrons
what happens when two atoms share electron pairs
When two atoms share pairs of electrons, they form covalent bonds
what is the strength level of covalent bonds?
covalent bonds between atoms are very strong
what happens when atoms are covalently bonded together
When two or more atoms are covalently bonded together, they form ‘molecules’
what 2 things can covalently bonded molecules consist of?
Covalently bonded substances may consist of small molecules or giant molecules
describe the reactivity of covalently bonded substances?
covalently bonded substances are very stable
what is present in between individual molecules?
weak intermolecular forces
describe the bonding and forces in methane (2)
in methane:
each molecule consists of four hydrogen atoms covalently bonded to a carbon atom
and in between individual methane molecules there are weak intermolecular forces
what are shared electrons known as
bonding electrons
what do bonding electrons occur in?
pairs
what are non bonding electrons?
Electrons on the outer shell which are not involved in the covalent bond(s) are called non-bonding electrons
why don’t simple molecules conduct electricity?
they don’t contain free electrons
2 shared electrons is equal to…
1 covalent bond
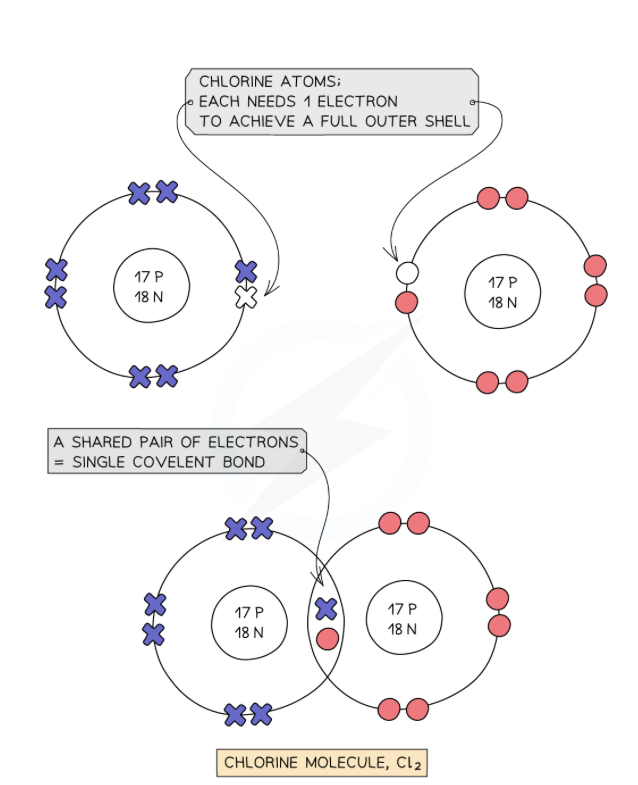
describe this diagram
there are 2 chlorine atoms
each chlorine atom has 7 electrons on their outermost shell and therefore need 1 electron each. this equates to the total need of 2 electrons
so they form 1 covalent bond with a shared pair of electrons forming a cl2 molecule
describe the differences between ionic and covalent bonding
covalent: shared pair of electrons, no ions formed
ionic: transferred electrons, ions formed