chemistry - rates of reaction & energy changes: heat energy changes in chemical reactions (7.9 - 7.16)
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
7.9 what changes do changes in heat energy accompany?
salts dissolving in water
neutralisation reactions
displacement reactions
precipitation reactions
7.9 how to show heat changes
measure temp. changes when reactions (prev. flashcard) take place in solution
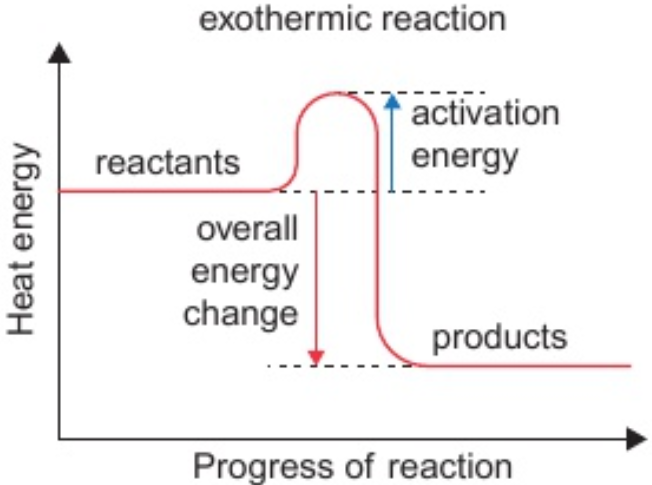
7.10 exothermic change/reaction
heat energy given out
(energy transferred from stores of energy in chemical bonds → surroundings)
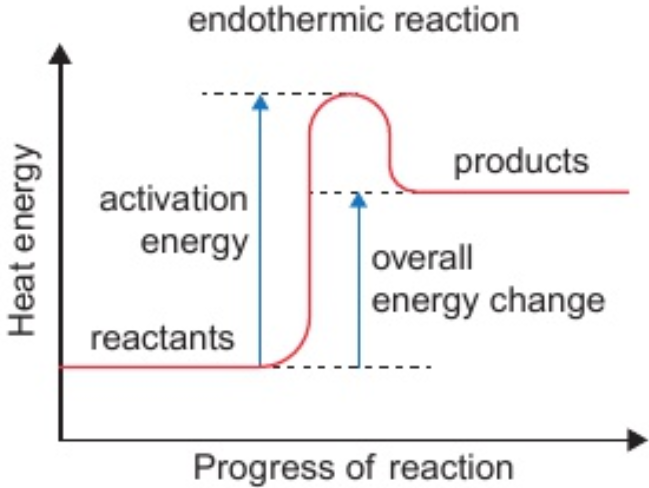
7.10 endothermic change/reaction
heat energy taken in
(energy transferred from surroundings → stores of energy in chemical bonds)
7.12 breaking bonds - endothermic/exothermic?
endothermic
7.12 making bonds - endothermic/exothermic?
exothermic
7.13 overall heat energy change for reaction is exothermic if:
more heat energy given out in making bonds in products than taken in in breaking bonds in reactants
7.13 overall heat energy change for reaction is endothermic if:
less heat energy given out in making bonds in products than taken in in breaking bonds in reactants
bond energy units
kJ mol-1
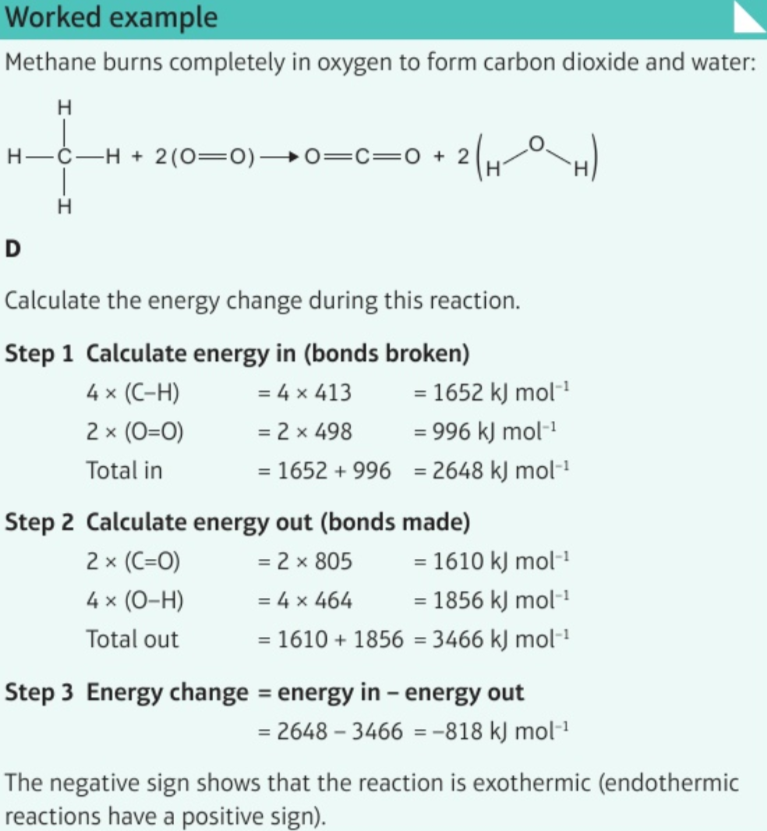
7.14 calculate energy change in reaction given energies of bonds
7.15 activation energy meaning
minimum amount of energy needed by colliding particles for reaction to occur
7.16 identify activation energy on reaction profile
from reactants line → highest point on curve
7.16 reaction profile - endothermic reaction
7.16 reaction profile - exothermic reaction