Virology 2 Exam 2
5.0(1)
Card Sorting
1/76
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
77 Terms
1
New cards
Lytic
replicate in the cell
form new progeny virions
form new progeny virions
2
New cards
How was phage therapy developed?
First evidence of phage:
* Found in the Ganges river in India, it was
* temperature sensitive
* capable of passing through a porcelain filter
* could reduce titres of the bacterium Vibrio cholerae in laboratory culture.
* Hankin suggested that it might help to decrease the incidence of cholera in people using water from the Ganges.
* Found in the Ganges river in India, it was
* temperature sensitive
* capable of passing through a porcelain filter
* could reduce titres of the bacterium Vibrio cholerae in laboratory culture.
* Hankin suggested that it might help to decrease the incidence of cholera in people using water from the Ganges.
3
New cards
Frederick Twort: co-discoverer of phage
* Twort was an English bacteriologist
* At the time, smallpox vaccines had to be made in the skin of calves and was almost always contaminated with Staphylococcus.
* In **1915**, Frank Twort, a British microbiologist, first observed glassy areas of colonies that would not grow when subcultured
* Not sure what they were but favored the idea that they were enzymes
* Research was disrupted by the outbreak of WWI and he never resumed study of phage
* At the time, smallpox vaccines had to be made in the skin of calves and was almost always contaminated with Staphylococcus.
* In **1915**, Frank Twort, a British microbiologist, first observed glassy areas of colonies that would not grow when subcultured
* Not sure what they were but favored the idea that they were enzymes
* Research was disrupted by the outbreak of WWI and he never resumed study of phage
4
New cards
First Virus
Tobacco Mosaic Virus 1880s
5
New cards
Félix d'Hérelle: co-discoverer of phage
* Was a French-Canadian microbiologist
* Not traditionally trained
* Helped stop a locust plague in Argentina and Mexico using pathogenic bacteria isolated from their own guts—biocontrol agent!
* During WWI, he moved to Paris to work in the Pasteur Institute, independently discovered phage Paris in 1917
* Not traditionally trained
* Helped stop a locust plague in Argentina and Mexico using pathogenic bacteria isolated from their own guts—biocontrol agent!
* During WWI, he moved to Paris to work in the Pasteur Institute, independently discovered phage Paris in 1917
6
New cards
Phage Isolation
* Neither knew exactly what phage were
* Not visible on microscopes at the time: electron microscopes not invented until 1933
* Phages not observed until 1939
* Published in German in 194 0
* Viruses are filterable agents
* Bacteria could be removed using a porcelain filter
* But viruses can pass through
* Not visible on microscopes at the time: electron microscopes not invented until 1933
* Phages not observed until 1939
* Published in German in 194 0
* Viruses are filterable agents
* Bacteria could be removed using a porcelain filter
* But viruses can pass through
7
New cards
Chamberlin Filter
Filtered out bacteria
8
New cards
How did Twort and d'Hérelle make their discoveries?
* Twort grew Staphylococcus in an agar slant
* He saw glassy clearings
* Subcultured: he tried picking that area and growing it in another slant but
* d'Hérelle grew dysentery bacillus
* Inoculate medium with bacteria, which grows and turns medium opaque
* Inoculate culture with phages, which kill the bacteria. The medium clears up
* Pass the culture through a porcelain filter, which removes the bacteria and allows the viruses to pass through. This filtrate can be used to kill bacteria as in the previous step
* He saw glassy clearings
* Subcultured: he tried picking that area and growing it in another slant but
* d'Hérelle grew dysentery bacillus
* Inoculate medium with bacteria, which grows and turns medium opaque
* Inoculate culture with phages, which kill the bacteria. The medium clears up
* Pass the culture through a porcelain filter, which removes the bacteria and allows the viruses to pass through. This filtrate can be used to kill bacteria as in the previous step
9
New cards
What is plaque?
they are voids in the bacterial lawn
10
New cards
The debate: what caused those clearings?
* Twort considered a microbe but favored that it was enzyme secreted by bacteria
* Work was almost forgotten
* d'Hérelle almost immediately concluded that they were a microbe that was feeding on the bacteria • Active debate in the field
* Jules Bordet, a microbiologist and Nobel Prize winner, rediscovered Twort’s work. Theorized that phages were enzymes already present in bacteria that could trigger the release of similar proteins, killing the bacteria
* Work was almost forgotten
* d'Hérelle almost immediately concluded that they were a microbe that was feeding on the bacteria • Active debate in the field
* Jules Bordet, a microbiologist and Nobel Prize winner, rediscovered Twort’s work. Theorized that phages were enzymes already present in bacteria that could trigger the release of similar proteins, killing the bacteria
11
New cards
d'Hérelle was controversial
* …so phage therapy also became controversial
* Despite this uncertainty, d'Hérelle using phages without consensus in the field on humans, his work was under constant attack from many other scientists
* Made enemies among powerful senior scientists, like Bardot
* was drummed out of the Pasteur Institute,
* held only one brief permanent position in the scientific establishment (at Yale University from 1928 to 1933)
* Was famous (and infamous) for self-advertisement, exaggerated claims of success and sharp financial practices
* Never won Nobel Prize, although nominated 10x
* Worked all over the world during a time of great division
* Despite this uncertainty, d'Hérelle using phages without consensus in the field on humans, his work was under constant attack from many other scientists
* Made enemies among powerful senior scientists, like Bardot
* was drummed out of the Pasteur Institute,
* held only one brief permanent position in the scientific establishment (at Yale University from 1928 to 1933)
* Was famous (and infamous) for self-advertisement, exaggerated claims of success and sharp financial practices
* Never won Nobel Prize, although nominated 10x
* Worked all over the world during a time of great division
12
New cards
Arrowsmith
* Published in 1925
* Won the Pulitzer Prize in 1926 Pulitzer Prize for Fiction, but Lewis declined the award
* Novel about the discovery of phage and using phage therapy to treat an outbreak of bubonic plague
* dealt with the culture of science
* Main character shares many biographical elements with d'Hérelle
* Won the Pulitzer Prize in 1926 Pulitzer Prize for Fiction, but Lewis declined the award
* Novel about the discovery of phage and using phage therapy to treat an outbreak of bubonic plague
* dealt with the culture of science
* Main character shares many biographical elements with d'Hérelle
13
New cards
Biocontrol agent:
a method of controlling pests, such as insects, mites, weeds, and plant diseases, using other organisms; relies on predation, parasitism, herbivory, or other natural mechanisms
\
using one organism to combat another organism you don’t want
\
using one organism to combat another organism you don’t want
14
New cards
d'Hérelle searched for biocontrol agents
* He’d find a bacterial disease that was causing an outbreak
* Look for phage in the feces of infected people
* Isolate the phage
* Treat infected people or animals with the phage to clear the infection
* In early 1919, isolated phages from chicken feces, successfully treating a plague of chicken typhus with them
* first human was healed of dysentery using phage therapy in August 1919
* Look for phage in the feces of infected people
* Isolate the phage
* Treat infected people or animals with the phage to clear the infection
* In early 1919, isolated phages from chicken feces, successfully treating a plague of chicken typhus with them
* first human was healed of dysentery using phage therapy in August 1919
15
New cards
Phages therapy started to gain traction
* d’Hérelle traveled the world to treating diseases, including India, Egypt
* Companies, including what would become L’Oréal and Eli Lilly, produced phage therapy cocktails • Used to treat cholera, dysentery, staphylococcus, the plague, streptococcus, E. coli, etc
* Oswaldo Cruz Institute in Rio de Janeiro, Brazil, started production of the antidysentery bacteriophages in 1924 to combat dysentery in Latin American countries
* \~1934, d‘Hérelle went to Tbilisi, Georgia
* welcomed to the Soviet Union as a hero
* Companies, including what would become L’Oréal and Eli Lilly, produced phage therapy cocktails • Used to treat cholera, dysentery, staphylococcus, the plague, streptococcus, E. coli, etc
* Oswaldo Cruz Institute in Rio de Janeiro, Brazil, started production of the antidysentery bacteriophages in 1924 to combat dysentery in Latin American countries
* \~1934, d‘Hérelle went to Tbilisi, Georgia
* welcomed to the Soviet Union as a hero
16
New cards
lysin
are hydrolytic enzymes produced by bacteriophages in order to cleave the host's cell wall during the final stage of the lytic cycle
\
Lysins are being used as antibacterial agents due to their high effectiveness and specificity
\
Lysins are being used as antibacterial agents due to their high effectiveness and specificity
17
New cards
Work in Tbilisi, Georgia
* d’Hérelle visited his friend Giorgi Eliava at the Tbilisi Bacteriology Laboratory in 1933-1934
* Was invited to the Soviet Union by Stalin
* Reportedly enamored by communism
* Could collaborate with Eliava
* Together they discovered bacteriophage lysins
* d’Hérelle intended to stay in Tbilisi permenantly
* Was invited to the Soviet Union by Stalin
* Reportedly enamored by communism
* Could collaborate with Eliava
* Together they discovered bacteriophage lysins
* d’Hérelle intended to stay in Tbilisi permenantly
18
New cards
Time in Tbilisi was brief
* Much of the research was published in Russian
* Even dedicated a book to Stalin but left shortly thereafter
* In 1937, Eliava, together with his wife Amelia Vol-Levitskaya (Polish opera singer), was arrested and executed as a ‘‘people’s enemy’’ for being in intellectual opposition with Laurenti Beria, the chief of the secret police
* Later renamed George Eliava Institute of Bacteriophage, Microbiology and Virology
* Even dedicated a book to Stalin but left shortly thereafter
* In 1937, Eliava, together with his wife Amelia Vol-Levitskaya (Polish opera singer), was arrested and executed as a ‘‘people’s enemy’’ for being in intellectual opposition with Laurenti Beria, the chief of the secret police
* Later renamed George Eliava Institute of Bacteriophage, Microbiology and Virology
19
New cards
d'Hérelle’s final years
* fled Tbilisi, never to return. His book was banned from distribution.
* returned to France
* Phage therapy boomed, despite all problems, driven by the military on both sides in an effort to keep the troops safe, at least from infections.
* was kept under house arrest by the German "Wehrmacht" in Vichy, France.
* used the time to write his book "The Value of Experiment” and his memoirs—800 pages long
* was stricken with pancreatic cancer and died a forgotten man in Paris in 1949
* returned to France
* Phage therapy boomed, despite all problems, driven by the military on both sides in an effort to keep the troops safe, at least from infections.
* was kept under house arrest by the German "Wehrmacht" in Vichy, France.
* used the time to write his book "The Value of Experiment” and his memoirs—800 pages long
* was stricken with pancreatic cancer and died a forgotten man in Paris in 1949
20
New cards
The Eliava Institute continued behind the Iron Curtain
* When the Georgian Civil War broke out in 1991, the Tbilisi facility was essentially ruined.
* facilities were damaged
* Thousands of samples catalogued in huge, refrigerated "libraries" suffered irreversible damage due to frequent electrical outages.
* Apparently, the Russians transferred some of the equipment to their territory and built plants for the production of phages in other locations.
* the Eliava Institute continued to deteriorate until it was on the verge of closure
* However, in 1997, a report on the institute was broadcast by the BBC, sparking a flurry of media interest in the West.
* Brought doctors, scientists, and entrepreneurs to Tbilisi
* facilities were damaged
* Thousands of samples catalogued in huge, refrigerated "libraries" suffered irreversible damage due to frequent electrical outages.
* Apparently, the Russians transferred some of the equipment to their territory and built plants for the production of phages in other locations.
* the Eliava Institute continued to deteriorate until it was on the verge of closure
* However, in 1997, a report on the institute was broadcast by the BBC, sparking a flurry of media interest in the West.
* Brought doctors, scientists, and entrepreneurs to Tbilisi
21
New cards
Iron Curtain
Soviet Union control
22
New cards
Why might phage therapy be more difficult to implement than antibiotics?
* antibiotics more general
* need specific bacteria for specific strain
* need specific bacteria for specific strain
23
New cards
Treatment and favorability of phages
* the new sulfa drugs of the 1930s, simple and well-characterized organic compounds, were easy to use, quite uniformly and dramatically effective against important infections, and widely available.
* Viruses are “at the borderline of life.”
* viruses could be crystallized, suggesting that they were “just” chemicals
* yet their ability to multiply and mutate suggests they were more than just chemicals
* Doctors tended to be solo practitioners without routine access to bacteriological laboratory resources
* Off-the-shelf medications and simple, locally compounded antibiotics were favored over phage therapy which required
* Viruses are “at the borderline of life.”
* viruses could be crystallized, suggesting that they were “just” chemicals
* yet their ability to multiply and mutate suggests they were more than just chemicals
* Doctors tended to be solo practitioners without routine access to bacteriological laboratory resources
* Off-the-shelf medications and simple, locally compounded antibiotics were favored over phage therapy which required
24
New cards
Implementation: phages vs antibiotics
* Lack of understanding of phage biology
* Phages are biological entities while antibiotics are chemicals
* More than just one type of phage infects a bacteria
* And they vary—culturing one is not necessarily like the other
* Require proper storage
* Phage have a narrower host range, so they are more precise killers
* But also harder to match the right phage for the infection
* Antibiotics tend to kill a broader spectrum of bacteria
* Phages are biological entities while antibiotics are chemicals
* More than just one type of phage infects a bacteria
* And they vary—culturing one is not necessarily like the other
* Require proper storage
* Phage have a narrower host range, so they are more precise killers
* But also harder to match the right phage for the infection
* Antibiotics tend to kill a broader spectrum of bacteria
25
New cards
The rise of antibiotics in the west
* After D-Day, the new antibiotic drug penicillin became public knowledge and became popular in the hospitals in the west.
* more reliable and easier to use than phage therapy, it soon became the method of choice, despite side effects and problems with resistant bacteria.
* Phage therapy remained a common treatment in the Soviet Union until its deconstruction.
* more reliable and easier to use than phage therapy, it soon became the method of choice, despite side effects and problems with resistant bacteria.
* Phage therapy remained a common treatment in the Soviet Union until its deconstruction.
26
New cards
Gunther Stent
* Born in Germany in 1924, emigrated to US in 1940
* Joined the ”Phage Group” at Cal Tech
* Staunch opponent of phage therapy
HATED PHAGE THERAPY
* Joined the ”Phage Group” at Cal Tech
* Staunch opponent of phage therapy
HATED PHAGE THERAPY
27
New cards
American Medical Association reports
* Meant to be a meta analysis but papers varied in terms of methods and materials,
* Some studies were therapeutic while others were prophylactic—but often not distinguished
* Not double-blinds trials used today
* First in 1934—published people at Yale
* Second in 1941—emphatically supported Bordet’s concept of phage as an auto-catalytically activated “lytic principle” in opposition to d’Herelle’s virus concept
* Third in 1945—in a complete reversal of prior dogma, fully accepted the viral nature of phage
* Published after EM of phage were disseminated
* Some studies were therapeutic while others were prophylactic—but often not distinguished
* Not double-blinds trials used today
* First in 1934—published people at Yale
* Second in 1941—emphatically supported Bordet’s concept of phage as an auto-catalytically activated “lytic principle” in opposition to d’Herelle’s virus concept
* Third in 1945—in a complete reversal of prior dogma, fully accepted the viral nature of phage
* Published after EM of phage were disseminated
28
New cards
Phage therapy was virtually forgotten in the West until the 1990s
The last commercial phage preparations disappeared from pharmaceutical markets in the early 1970s.
29
New cards
Why is there renewed interest in phage therapy?
Antibiotic resistance
rise of the superbugs (multi antibiotic resistance)
rise of the superbugs (multi antibiotic resistance)
30
New cards
Who discovered phages?
* Phage first observed by British microbiologist Twort
* Félix d'Hérelle independently in the Pasteur Institute in Paris in 1917 Immediately thought they were viruses
\
* Félix d'Hérelle independently in the Pasteur Institute in Paris in 1917 Immediately thought they were viruses
\
31
New cards
Why was d'Hérelle controversial?
Despite this uncertainty, d'Hérelle using phages without consensus in the field on humans, his work was under constant attack from many other scientists Made enemies among powerful senior scientists, like Bardot
32
New cards
Biological reasons that phages were historically more difficult use for treatment
* Lack of understanding of phage biology
* Phages are biological entities while antibiotics are chemicals
* More than just one type of phage infects a bacteria
* And they vary—culturing one is not necessarily like the other
* Require proper storage (sometimes)
* Phage have a narrower host range, so they are more precise killers But also harder to match the right phage for the infection
* Phages are biological entities while antibiotics are chemicals
* More than just one type of phage infects a bacteria
* And they vary—culturing one is not necessarily like the other
* Require proper storage (sometimes)
* Phage have a narrower host range, so they are more precise killers But also harder to match the right phage for the infection
33
New cards
World Health Organization declared that antibiotic resistance to be one of the biggest threats to
global health, food security, and development
34
New cards
How does antibiotic resistance occur?
1. High number of bacteria. A few of them are resistant to antibiotic
2. Antibiotics kill bacteria causing the illness, as well as good bacteria protecting the body from infection
3. The resistance bacteria now have preferred conditions to grow and take over
4. Bacteria can even transfer their drug-resistance to other bacteria, causing more problems
35
New cards
Why are people again interested in phage therapy?
Antibiotic resistance is on the rise!
36
New cards
Modern applications of phage therapy
* Food safety
* Personalized, compassionate-use cases
* Rare
* Expensive
* Clinical trials
* Personalized, compassionate-use cases
* Rare
* Expensive
* Clinical trials
37
New cards
What foods are risky?
* sprouts
* raw milk
* soft cheese
* deli meats and hot dogs
* smoked seafood
* raw milk
* soft cheese
* deli meats and hot dogs
* smoked seafood
38
New cards
Food safety
Phages have increasingly been used to make food products safer to consume and to forestall spoilage bacteria
39
New cards
T/F Phages harbor great biodiversity
True
40
New cards
Biodiversity and phage specificity
* Phage are more specific killers than antibiotics
* experimental demonstration that one or more phages can infect the specific bacterial strain causing the infection, at least in vitro
* assumes that efficient phage killing of the bacteria in lab will also occur in patient
* prescreening is critical because bacterial isolates can vary enormously in their phage infection profiles
* rare to confidently predict that one or more phages will infect the bacterial clinical isolate without prior testing
* experimental demonstration that one or more phages can infect the specific bacterial strain causing the infection, at least in vitro
* assumes that efficient phage killing of the bacteria in lab will also occur in patient
* prescreening is critical because bacterial isolates can vary enormously in their phage infection profiles
* rare to confidently predict that one or more phages will infect the bacterial clinical isolate without prior testing
41
New cards
3 seminal case studies
* Approved by FDA under emergency Investigational New Drug (eIND) between 2017-2019
* traditional antimicrobial therapy had failed
* phages directed at specific infectious organisms utilized in combination with antibiotics
* All were associated with successful microbiological and clinical responses
* traditional antimicrobial therapy had failed
* phages directed at specific infectious organisms utilized in combination with antibiotics
* All were associated with successful microbiological and clinical responses
42
New cards
Phage cocktails
* a case of necrotizing pancreatitis
* disseminated Acinetobacter baumannii infection
* near death after 5 months of failed conventional antibiotic therapy
* disseminated Acinetobacter baumannii infection
* near death after 5 months of failed conventional antibiotic therapy
43
New cards
Problem: bacteria can evolve resistance to phage
* Solution: use phage that drive trade-offs for resistance to antibiotics and then use in combination with antibiotics!
44
New cards
Choosing phage that force trade-offs
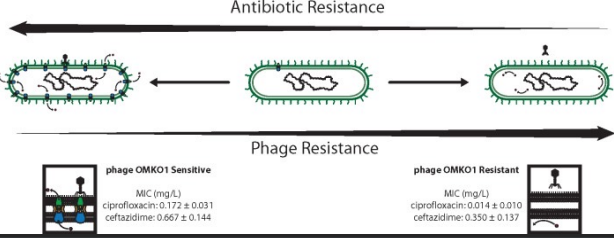
* Chose phage (OMKO1) the attach to efflux pumps. This drives Pseudomonas aeruginosa to become susceptible to antibiotic (ciprofloxacin)
* Also treat with antibiotic—bacteria can evolve resistance to both phage and antibiotics simultaneously!
* Also treat with antibiotic—bacteria can evolve resistance to both phage and antibiotics simultaneously!
45
New cards
Problem: some phage have large genomes that have a variety of functions that may not be beneficial during phage therapy
Solution: Engineered phages used to remove undesirable genes before application
46
New cards
How are phages collected?
* can be collected from environmental sources
* sources that likely contain high quantities of bacteria associated with humans and their bacteriophages
* effluent outlets
* sewage
* soil
* sources that likely contain high quantities of bacteria associated with humans and their bacteriophages
* effluent outlets
* sewage
* soil
47
New cards
Which playa lake would you focus on to collect phages in Lubbock and why?
Max lake- behind a hospital
48
New cards
Assessing phage therapy candidates
* Grown on bacterial hosts
* Screened in lab for desirable characteristics
* Can grow on clinical strains
* Screened in lab for desirable characteristics
* Can grow on clinical strains
49
New cards
Desirable properties of therapeutic phages
1. the phages should kill the specific bacterial pathogen efficiently in vitro, without significant levels of bacterial survival
2. the phages should be easy to propagate, to produce in high titer preparations, and to purify
3. the phages should be stable over a range of concentrations, such that storage for extended time periods at refrigerator temperatures is not associated with substantial loss of infectivity.
4. the phage preparations must be sterile and free of endotoxins or other harmful contaminants
5. the phage genomes should not include any genes known or suspected to be toxic.
6. the phages should not be able to act as generalized transducing phages
50
New cards
T/F Bacteria can evolve resistance to phage
True
51
New cards
What is a phage cocktail?
* treating an infection with more than one type of phage
* Cocktails of more than one phage can help to minimize phage resistance.
* if two phages in a cocktail, it is advisable that they do not use the same cellular receptor, as receptor loss will confer resistance to both phages.
* Cocktails of more than one phage can help to minimize phage resistance.
* if two phages in a cocktail, it is advisable that they do not use the same cellular receptor, as receptor loss will confer resistance to both phages.
52
New cards
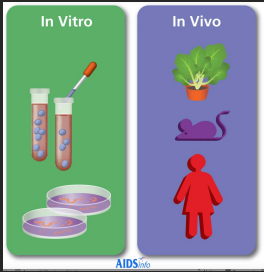
Phages in vitro vs in vivo
* Phage may behave in vitro and in the patient quite differently.
* In vivo, bacteria may form biofilms, may live in hypoxic or intracellular environments in which the phage particles either have poor access to their hosts or do not kill them efficiently.
* Further studies are needed to understand how these differences lead to poor clinical outcomes.
* In vivo, bacteria may form biofilms, may live in hypoxic or intracellular environments in which the phage particles either have poor access to their hosts or do not kill them efficiently.
* Further studies are needed to understand how these differences lead to poor clinical outcomes.
53
New cards
Host immune response to phage therapy
* You already have phages naturally in your microbiome
* Immune reactions to the phages typically not a substantial concern
* Possibility of immune reactions that interfere with phage function with some routes of administration especially intravenously and should be studied
* May want to consider
* Administering phages sequentially rather than together in a cocktail to extend the potential treatment regimen.
* map the landscape of immunological cross-reactivity for phages of any particular pathogen, such as to determine whether a neutralizing reaction to one phage inactivates others.
* In some extreme clinical circumstances, administration of immunosuppressive drugs with the phages
* Immune reactions to the phages typically not a substantial concern
* Possibility of immune reactions that interfere with phage function with some routes of administration especially intravenously and should be studied
* May want to consider
* Administering phages sequentially rather than together in a cocktail to extend the potential treatment regimen.
* map the landscape of immunological cross-reactivity for phages of any particular pathogen, such as to determine whether a neutralizing reaction to one phage inactivates others.
* In some extreme clinical circumstances, administration of immunosuppressive drugs with the phages
54
New cards
What is the ELIAVA Institute?
The George Eliava Institute of Bacteriophage, Microbiology and Virology has been active since the 1930s in the field of phage therapy, which is used to combat microbial infection.
55
New cards
Superbugs
resistant antibiotics
56
New cards
Why are people again interested in phage therapy?
rise in antibiotic resistance
57
New cards
ESKAPE pathogens
* 6 clinically- relevant, highly virulent and antibiotic-resistant bacterial pathogens
* one of the largest global health challenges
* Gram-positive and Gram-negative bacteria can evade or 'escape' commonly used antibiotics
* one of the largest global health challenges
* Gram-positive and Gram-negative bacteria can evade or 'escape' commonly used antibiotics
58
New cards
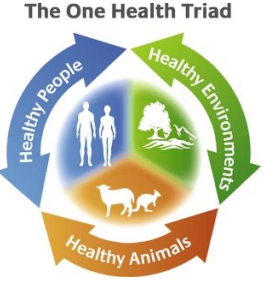
One Health
* the unity of multiple practices that work together locally, nationally, and globally to help achieve optimal health for people, animals, and the environment
* combatting the highly resistant and opportunistic ESKAPE pathogens necessitates a One Health approach
* combatting the highly resistant and opportunistic ESKAPE pathogens necessitates a One Health approach
59
New cards
prophylactic
60
New cards
Enterococcus faecium
* can be commensal in the gastrointestinal tract of humans and animals
* may also be pathogenic,
* neonatal meningitis
* endocarditis
* often exhibits a resistance to β-lactam antibiotics, including penicillin and other last resort antibiotics
* rise in vancomycin resistant enterococci (VRE) strains
* the thicker biofilms act as a “mechanical and biochemical shield”
* may also be pathogenic,
* neonatal meningitis
* endocarditis
* often exhibits a resistance to β-lactam antibiotics, including penicillin and other last resort antibiotics
* rise in vancomycin resistant enterococci (VRE) strains
* the thicker biofilms act as a “mechanical and biochemical shield”
61
New cards
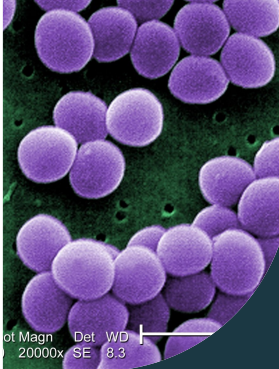
Staphylococcus aureus
* commonly found as a part of the human skin microbiota and is typically not harmful in humans with non-compromised immune systems
* Can cause infections when it enters parts of the body that it does not typically inhabit, such as wounds
* can also cause infections on implanted medical devices and form biofilms that make treatment with antibiotics more difficult
* Methicillin-resistant S. aureus, or MRSA, includes strains that have developed resistance to βlactam antibiotics
* Can cause infections when it enters parts of the body that it does not typically inhabit, such as wounds
* can also cause infections on implanted medical devices and form biofilms that make treatment with antibiotics more difficult
* Methicillin-resistant S. aureus, or MRSA, includes strains that have developed resistance to βlactam antibiotics
62
New cards
Klebsiella pneumoniae
* **particularly adept to accepting resistance genes in horizontal gene transfer**
* **Certain strains have also developed βlactamases that allow them to be resistant many of the commonly used antibiotics, including carbapenems**
* implicates plasmids as the primary source of the resistance genes
* ability to produce extended-spectrum betalactamases (ESBL) are resistant to virtually all beta-lactam antibiotics, except carbapenems
* **Certain strains have also developed βlactamases that allow them to be resistant many of the commonly used antibiotics, including carbapenems**
* implicates plasmids as the primary source of the resistance genes
* ability to produce extended-spectrum betalactamases (ESBL) are resistant to virtually all beta-lactam antibiotics, except carbapenems
63
New cards
Acinetobacter baumannii
* common in hospitals, which has allowed for the development of resistance to all known antimicrobials
* thrives in unaccommodating environments due to its tolerance to a variety of temperatures, pHs, nutrient levels, as well as dry environments
* efflux pump and outer membrane, allow a wider range of antibiotic resistance
* thrives in unaccommodating environments due to its tolerance to a variety of temperatures, pHs, nutrient levels, as well as dry environments
* efflux pump and outer membrane, allow a wider range of antibiotic resistance
64
New cards
Pseudomonas aeruginosa
* able to survive in extreme environments as well as in soil and many more common environments
* survives quite well in the lungs of patients with late-stage cystic fibrosis
* Can express β-lactamases as well as upregulated efflux pumps which can make treatment particularly difficult
* survives quite well in the lungs of patients with late-stage cystic fibrosis
* Can express β-lactamases as well as upregulated efflux pumps which can make treatment particularly difficult
65
New cards
Enterobacter spp.
* Some strains cause urinary tract (UTI) and blood infections
* Colistin and tigecycline are two of the only antibiotics currently used for treatment
* Colistin and tigecycline are two of the only antibiotics currently used for treatment
66
New cards
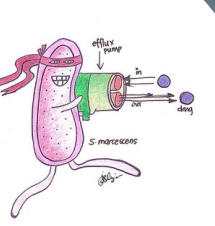
Efflux pumps
* Large impact on antimicrobial resistance
* genetic elements encoding efflux pumps may be encoded on chromosomes and/or plasmids **So**
* efflux pump genes can survive a hostile environment (for example in the presence of antibiotics) which allows for the selection of mutants that over-express these genes.
* genetic elements encoding efflux pumps may be encoded on chromosomes and/or plasmids **So**
* efflux pump genes can survive a hostile environment (for example in the presence of antibiotics) which allows for the selection of mutants that over-express these genes.
67
New cards
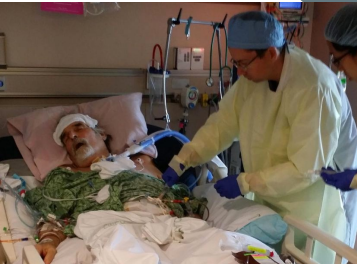
MDR A. baumanni infection
* In 2016, Tom Patterson fell sick after complications with a gall stone while traveling in Egypt
* had a case of necrotizing pancreatitis Disseminated multidrug resistant Acinetobacter baumannii infection
* near death after 5 months of failed conventional antibiotic therapy
* His wife, Steffanie Strathdee, was the Associate Dean of Global Health Sciences at UC San Diego
* enlisted the help of an international team of physicians and researchers
* had a case of necrotizing pancreatitis Disseminated multidrug resistant Acinetobacter baumannii infection
* near death after 5 months of failed conventional antibiotic therapy
* His wife, Steffanie Strathdee, was the Associate Dean of Global Health Sciences at UC San Diego
* enlisted the help of an international team of physicians and researchers
68
New cards
Individualized phage therapy
* Applied for compassionate care usage of phage
* Contacted labs around the US and created 2 cocktails with 4 phage each
* Contacted labs around the US and created 2 cocktails with 4 phage each
69
New cards
Mixing the cocktail
* Screen 200 A. baumannii-specific lytic bacteriophages were screened for activity against the three clinical isolates in this study
\
**Phages were previously harvested from environmental sources by the Biological Defense Research Directorate (BDRD) of the Naval Medical Research Center (NMRC) were screened for activity against the three clinical isolates in this study**
* A subset of 98 were highly active against a broad range of MDR A. baumannii isolates
\
**Phages were previously harvested from environmental sources by the Biological Defense Research Directorate (BDRD) of the Naval Medical Research Center (NMRC) were screened for activity against the three clinical isolates in this study**
* A subset of 98 were highly active against a broad range of MDR A. baumannii isolates
70
New cards
Screening of A. baumannii phage library against TP1 clinical isolate
* media changes color indicates bacteria growing
71
New cards
**Problem**: phage tend to be more specific than antibiotics, so may be difficult to identify a phage effective at
**Solution:** use phage cocktails, or combinations of phages, to help ensure that phages
72
New cards
Bacteriophage titer from plasma samples during bacteriophage therapy
* Plasma samples collected 5 min prior to and following administration of 5 × 109 PFU of bacteriophage via intravenous injection
* indicated that bacteriophage titers in systemic circulation increase rapidly
* The bacteriophage titer dropped 120 min postinjection
* no detectable bacteriophage titer 6 hours after injection
* indicated that bacteriophage titers in systemic circulation increase rapidly
* The bacteriophage titer dropped 120 min postinjection
* no detectable bacteriophage titer 6 hours after injection
73
New cards
**Problem**: bacteria can evolve resistance to phage
**Solution**: use phage that drive trade-offs for resistance to antibiotics and then use in combination with antibiotics!
74
New cards
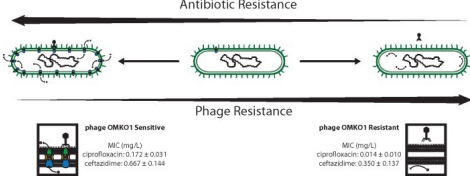
Choosing phage that force trade-offs
* Chose phage (OMKO1) the attach to efflux pumps. This drives Pseudomonas aeruginosa to become susceptible to antibiotic (ciprofloxacin)
* Also treat with antibiotic—bacteria can evolve resistance to both phage and antibiotics simultaneously!
* Also treat with antibiotic—bacteria can evolve resistance to both phage and antibiotics simultaneously!
75
New cards
How a virus can reverse antibiotic resistance:
1. some types of bacteria can resist antibiotics by using pumps to flush out the drugs before they can cause damage
2. these bacteria can be infected by certain viruses that bind to the pumps
3. the viruses replicate inside the bacteria, killing them in the process. some bacteria mutate and stop making the pumps, resisting the viruses
4. the remaining mutant bacteria can be killed with antibiotics
76
New cards
Selection for phage resistance restores antibiotic sensitivity
* Selection for phage resistance causes a trade-off resulting in significantly reduced Minimum Inhibitory Concentrations (MIC) to four drugs drawn from different antibiotic classes.
* LEFT: Average MIC ± SD of four antibiotics for phage sensitive MDR bacteria (left column) and for spontaneous mutants of these bacteria resistant to phage OMKO1 (right column).
* RIGHT: Fold improvement of MIC for isolated strains resistant to OMKO1 (\*p < 0.05, \*\*p < 0.01). For comparison, data for fold-increased sensitivity of transposon knockout PAO1-∆oprM (phage resistant) is displayed as a vertical black line
* LEFT: Average MIC ± SD of four antibiotics for phage sensitive MDR bacteria (left column) and for spontaneous mutants of these bacteria resistant to phage OMKO1 (right column).
* RIGHT: Fold improvement of MIC for isolated strains resistant to OMKO1 (\*p < 0.05, \*\*p < 0.01). For comparison, data for fold-increased sensitivity of transposon knockout PAO1-∆oprM (phage resistant) is displayed as a vertical black line
77
New cards
**Problem**: some phage have large genomes that have a variety of functions that may not be beneficial during phage therapy
**Solution**: Engineered phages used to remove undesirable genes before application
* 15yr old with cystic fibrosis: infection with Mycobacterium persisted through double lung transplant
* 15yr old with cystic fibrosis: infection with Mycobacterium persisted through double lung transplant