lesson 3: solubility equilibria
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
74 Terms
Chemical equilibrium
State in which the rates of the forward and reverse reactions are equal. Concentrations remain constant.
Equilibrium
Is a dynamic process and there is no net change in the number of molecules of reactants or products.
Equilibrium expression
Products raised to coefficients (if any) over reactants raised to coefficients (if any)
aA + bB ⇆ cC + dD; where small letters are the coefficient
Equilibrium constant allows us to predict:
The extent of reaction when equilibrium is established.
The direction of net reaction from a given set of initial concentrations.
Whether a given set of concentrations represents an equilibrium condition.
K > 1 where K is equilibrium constant
Reactant favored
K = 1 where K is equilibrium constant
Value of K at equilibrium
K < 1 where K is equilibrium constant
Product favored
Equilibrium constant for new reaction
Inverse of the original reaction
Homogenous equilibrium
All products and reactants are in the same physical state.
Heterogenous equilibrium
Reactants and products in more than one phase. Does not depend on the amounts of pure solids or liquids.
Le Châtelier's Principle
States that if an external stress (temperature, pressure, concentration, volume) is added to a system, the equilibrium shifts to a new position.
Concentration - equilibrium
Addition of reactant shifts the equilibrium to the right
Addition of products shifts the equilibrium to the left
USE SEASAW METHOD
Pressure - equlibrium
Increase shifts the equilibrium to the fewer gas molecules.
Decrease shifts the equilibrium to the side with more gas molecules
Increase in pressure - pressure equilibrium
Equilibrium shifts in reverse
Favors the side of fewer moles of gas
Increase in temperature - equilibrium
Exothermic reaction (heat released, system absorbs heat)
Favors reverse reactions
ONLY FACTOR THAT HAS EFFECT ON K
Decrease in temperature - equilibrium
Endothermic reaction (heat absorbed, system releases heat)
Favors forward reactions
ONLY FACTOR THAT HAS EFFECT ON K
Catalysts
Speed up or retard the rate of equilibrium.
Ksp
Equilibrium constant for a sparingly soluble salt
Lower = more soluble
Reaction quotient
Also called the ion product. Gives the same ratio the equilibrium expression gives, but for a system that is not at equilibrium.
Q = K
System is at equilibrium state
Q > K
Too much product (precipitate will form), equilibrium shits to the reactant side
Q < K
Too much reactant (unsaturated solution), equilibrium shifts to the product side.
Common ion effect (increase in common ion decreases in solubility)
Solubility of sparingly soluble species is reduced. Inverse relationship with solubility. A salt is less soluble if one of its __ is already present in the solution.
Effect of pH
Sparingly soluble from weak acids tend to be more soluble in an acidic solution. Inverse relationship with solubility. More acidic = more soluble
Effect of complexation (ion pairs)
Formation of complex ions increases the solubility of a sparingly soluble salt. Directly proportional relationship with solubility.
Salt effect
Diverse ion increase the Ksp value. In the presence of an inert electrolyte, activities of ions decrease. Adding an inert electrolyte increases the solubility of a sparingly soluble salt. Directly proportional relationship with solubility.
Electrolytes
Create an ionic atmosphere that decreases the attraction between ions in the solution.
Effect of electrolytes
To reduce the tendency for salts to come together thereby increasing solubility.
Ionic strength
Measure of total concentrations of ions in a solution.
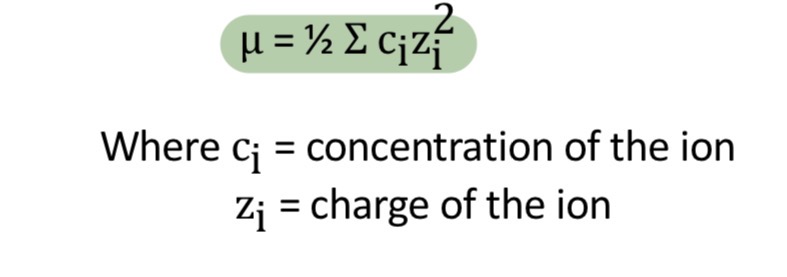
Gilbert Newton Lewis
Introduced the concept of activity and formed a model in acids and bases where electrons are involved.
Activity coefficient
Measures the deviation of behavior from ideality. If it’s 1, behavior is ideal and first equation would be correct.
Peter Debye and Erich Huckel
Quantified that an expression permits the calculation of activity.
Interpolation
Estimation of a number that lies between two values in a table.
Higher charge
The lower the activity coefficient (__ and coefficient have inversely proportional relationship).
Higher ionic strength
The lower the activity coefficient (__ and coefficient have inversely proportional relationship).
Salting out effect
Increase of activity coefficients with ionic strength (𝜇≥ 1M)
Svante August Arrhenius
Swedish chemist proposed two classification of compounds; acids and bases.
Arrhenius acid
Increases the concentration of hydronium ions (H+)
Arrhenius base
Increases the concentration of hydroxide ions (OH-)
Thomas Martin Lowry and Johannes Nicolaus Brønsted (Brønsted-Lowry theory)
Proton theory of acids and bases. Introduced independently in 1923.
Brønsted-Lowry acid
Proton donor
Brønsted-Lowry base
Proton acceptor
Conjugate acid
Species that forms after a base accepts a proton
Conjugate base
Species that forms after an acid donates a proton
Lewis acid
Electron pair acceptor
Lewis base
Electron pair donor
Adduct
Compound with coordinate covalent bond, both electrons are provided by only one of the atoms.
Ion product constant of H2O at 25°C
Kw = 1.0×10^-14 M
Calculating for pH
= -log[H+]
= -log[A_H+]
= -log[H+]γ_H+
= 14 - pOH or 14 - (-log[OH-])
Strong acids and bases
Species that completely ionize in water
Weak acid and weak base or weak electrolyte
Partially ionized (<5%) in aqueous solution.
Stronger acid
Product is weaker conjugate base
Weaker acid
Product is stronger conjugate base
Equilibrium constant expression for acid
Lower equilibrium constant for this, the weaker it is.
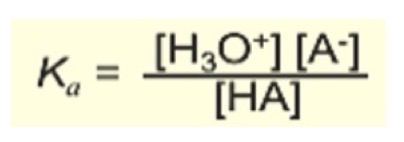
Percent ionization
If less than 5%, ionization is negligible.

Stronger base
Product is weaker conjugate base
Weaker base
Product is stronger conjugate acid
Equilibrium constant expression for base
Lower equilibrium constant for this, the weaker it is.

Salt that produce neutral solutions
From strong bases and strong acids, they do not hydrolyze and remain neutral at 7.
Hydrolysis of salt in basic solutions
Cation of a strong base + anion of a weak acid. Anion will accept proton from the water.
Hydrolysis of salt in acidic solutions
Cation of a weak base + anion of a strong acid. Cation is a weak acid that will form H3O+
Hydrolysis of salt
If both anion and cation can react with water, the solution will depend on the greater equilibrium constant value. (i.e. ka > kb, meaning solution is acidic)
Monoprotic acid
Contains one proton
Diprotic acid
Contains two protons. The first proton is the easiest proton removed compared to the successive proton.
Triprotic acid
Contains three protons. The first proton is the easiest proton removed compared to the successive proton.
Polyprotic acid
Contains more than one proton. The first proton is the easiest proton removed compared to the successive proton.
Amphoteric or amphiprotic
A substance that can react as both acid or base.
Buffer solutions
Resist change in pH on a small dilution
Contains a weak acid or weak base and their salts
Buffering capacity (BC)
Measure of the resistance of a buffer to pH.
Maximum buffering capacity
When concentration of base is equal to concentration of acid.
pH calculation of buffer, Henderson Hasselbalch equation
In choosing a buffer, seek one whose pKa is as close as possible to the desired pH.
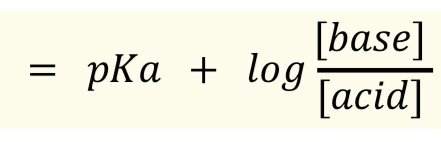
Charge balance
Sum of positive charges equals the sum of the negative charges in a solution.
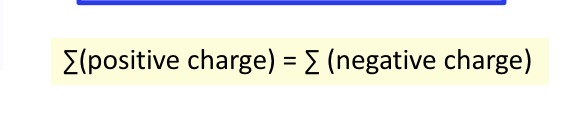
Mass balance
Quantity of all species in a solution containing a particular atom must equal to the amount of that atom delivered in a solution.

Systematic treatment of equilibrium
Write as many independent equations as there are chemical species in the system. Includes:
equilibrium constant expressions
Mass balance equations
Single charge balance equation