Wildlife Disease Final
1/70
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
71 Terms
Define a microbiome
Collection of microbes that live in and on our bodies
Composed of a large number of diverse organisms
Name two body systems that are linked to the gut microbiome
Digestion / Nutrition
Nervous system
Endocrine system
Cardiometabolic system
Immune system
Name two model animals used to study human microbiomes
Lab mice
Zebrafish
Which microbiome and infectious disease was analyzed in the example with red foxes, grey foxes and coyotes?
Skin microbiomes
Sarcoptic mange
Why would microbiomes be considered vital for conservation reintroductions?
Name a species where this was shown to be the case.
Captive reared individuals had vastly different diets and microbiomes to those of their wild conspecifics
Could impact fitness & their ability to ward off infection
Affect reintroduction success
Amargosa vole
What are the proximate (most recent) and ultimate causes of disease emergence?
Proximate = rising temperatures (ecological driver)
Ultimate causes = poverty, politics (non-ecological drivers)
What are the four main drivers of disease emergence?
Globalization
Climate Change
Land Use Change
Urbanization
What disease was an example of travel driving a pandemic?
ZIKA virus / COVID-19
Give an example of how wildlife trade drives the spread of infectious disease.
Meat Markets and Leptospirosis infections in Laos
Chytridiomycosis / Ranavirus
Burmese pythons are an example of what form of globalization?
Explain how they are responsible for the spread of which disease?
Invasive alien species
Ate all the larger mammals and left the hisipid cotton rats which are reservoirs for Everglades virus (mosquito-borne)
What is the cause of the Ghost Moose?
Climate change increasing winter temperatures - leading to large numbers of ticks on moose and causing fur loss
What is this image depicting? Explain your answer.
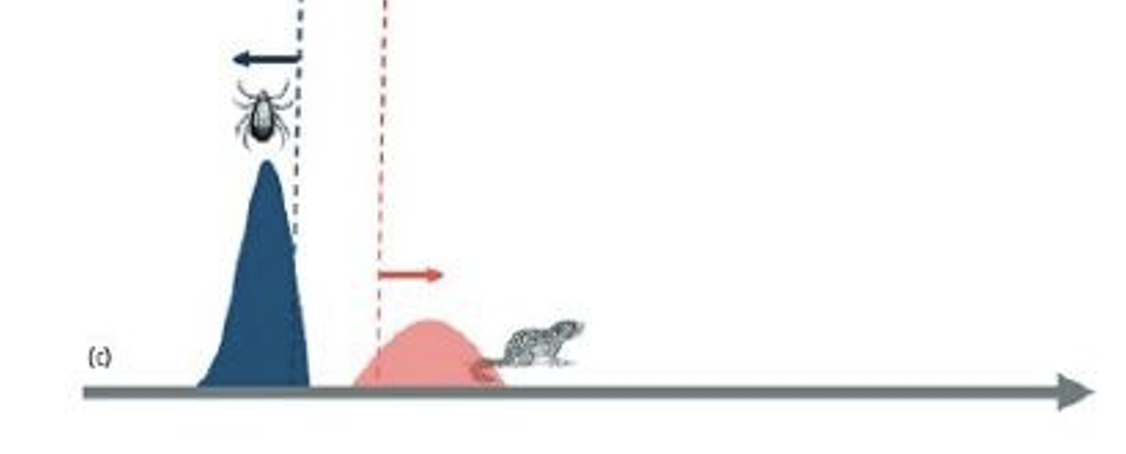
Ecological mismatch
increased asynchrony in the phenology of vector and host disrupts pathogen transmission
In 2006, Norway lost 20% of their muskoxen to a disease outbreak.
What was the cause of disease emergence and what type of pathogen was responsible?
Extreme weather event, Unusually high winter temperatures
Caused bacterial infection - pasteurellosis
Give an example of a disease emergence based on land use change.
Name the reservoir species and affected host species.
Hendra virus
Loss of Eucalyptus trees led to shifting distributions of flying foxes
Spread disease to horses and humans
In Flagstaff, rabies outbreaks showed transmission from bats to skunks. How did urbanization play a role in disease spread?
Skunks found living in and around houses where bats occur
Skunks are either bitten by bats or consumed the dead infected bats
People putting out bird and cat feeders increased the number of skunks and other mesocarnivores visiting a site - increasing transmission risk
What is the term for a species that can become infected, sustain transmission and can serve as a source of infection?
Reservoir species
What does NT stand for and how is it used in disease control?
Threshold population for invasion (NT): the minimum host population size required for a disease to be able to invade a host population successfully
Control efforts typically aim to reduce the susceptible population to below NT using policies based on culling, sterilization and/or vaccination
What type of disease intervention was used for the control of rabies in foxes in Belgium?
Vaccination of reservoir species
What are the 4 ways in which we can manage diseases in wildlife populations?
Do nothing
Reduce disease in reservoir species
Reduce disease in target species
Reduce transmission between target and reservoir species
When controlling parasites in host populations, which of the following factors of R0 would you try to affect?
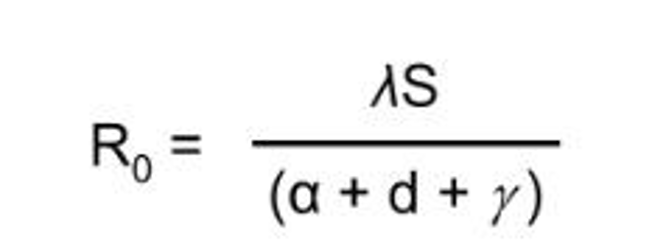
Decrease 𝛌
Decrease the susceptible population
Increase 𝜸
Why was culling of badgers to control bovine Tb unsuccessful in the UK?
Culling changed the badgers' behavior where they moved to the surrounding areas.
When attempting to limit reproduction in reservoirs, what should you try to minimize?
Reduce behaviors that leads to contact to minimize contact among individuals
What is the difference between resistance and tolerance?
RESISTANCE : An organism’s ability to limit the level of infection
Vaccination / treatments
TOLERANCE : An organism’s ability to survive, reproduce, and grow with a given infection
Food supplementation / reducing predation pressure etc.
What are some of the problems associated with vaccinating target species?
Very few follow-ups to determine effectiveness and weather vaccination leads to higher survival
CRISIS situation so usually vaccinate all individuals (no controls for scientific rigor)
Treatment of target species is often problematic. Why is this? Give an example of a successful treatment program (species and disease)
Effectiveness has rarely been assessed
Handling stress may outweigh benefit of treatment
Ongoing commitment
Examples: Red fox and E. multilocularis (tapeworm) - Japan
Koalas and chlamydia - Australia Wombats and sarcoptic mange - Australia
For which of the following diseases is culling of the target animals considered as a control measure?
Sea Star Wasting Disease
Chronic Wasting Disease
Valley Fever
Chronic Wasting Disease - culling large adult males reduced disease prevalence BUT problems with hunters…
What was the disease that spread to Arizona’s bighorn sheep from the introduction of domestic goats?
Infectious Keratoconjunctivits
What are the links between health and microbiomes?
Gut microbiome = most abundant, diverse microbial ecosystem in human body
Microbiomes contribute to homeostasis (Modulates nutrition, Modifies immune status, Protects against infection, Detoxifies chemicals, Impacts behavior)
Microbiomes studies use mouse and zebrafish models, mouse gut microbiome experiment that showed that gut microbiomes are linked to being lean or obese
Explain bee and intestinal parasite example
■ Bee and intestinal parasite example: Adult honeybees and bumble bees harbor a specialized and surprisingly species-poor community of bacteria in
their gut, These bacteria appear absent in solitary bees, suggesting an association with sociality, The specialized gut microbiota of bumble bees does protect them against infection by C. bombi But exposure to feces from nest mates was needed after pupal eclosion for successful establishment of these microbiota and to provide the protective effect
Explain the links between urbanization, diet and microbiomes in American White Ibis
Urban land cover was significantly
associated with shifts in the birds' gut
microbiome
• diet (especially diets involving
provisioned food) playing a lesser
role
• Gut microbial diversity
• NEGATIVELY impacted by urban land
cover
• Significant decrease with increasing
disease prevalence
KEY FINDING: Urban environments and dietary changes
can alter gut microbiome diversity
• influence health and pathogen vulnerability in wildlife
as they adapt to urban habitats
What is the take-home message from the mange and skin microbiomes in mesocarnivores example
Across species:
• consistent signatures of mange infection
• reduced skin microbiome diversity
• shifted community composition
• Mange infection status was the primary driver
of microbial community structure
What are the main wildlife conservation threats and give an example of each.
Land-use change: Udzungwa red colobus monkey, threatened primate species, Primarily arboreal, Highly sensitive to hunting and habitat destruction, Two forests – disturbed vs. undisturbed, Gut bacterial diversity HIGHER in undisturbed forest
Environmental contamination: Honeybees exposed to pesticides have microbiomes depleted of microbes involved in sugar metabolism and protease activities, which are vital to nectar processing, possibly having downstream effects on honeybee health
Climate change: Corals stressed by warming and acidifying ocean conditions release antibacterial compounds which could impact the diversity and composition of their associated microbiomes
Disease: Western lowland gorillas, Central African Republic, GI parasites and gut microbiome, Entamoeba spp. infections were associated with significant differences in abundances of bacterial taxa that likely play important roles in nutrition and metabolism for the host
What are the conservation implications of captivity on microbiome composition? Give an example and how this could affect reintroduction success.
Amargosa vole: Captive reared individuals had vastly different diets and microbiomes to those of their wild conspecifics
• Could impact fitness
• Could impact their ability to ward off infection
• Affect reintroduction success
What are the three main ways in which travel can lead to emerging infectious diseases?
Travel brings zoonotic infections to non-endemic areas (Marburg virus, cave of bats tourists bringing disease to Amsterdam), Travel drives stuttering outbreaks in non-endemic areas (Researchers studying malaria brought back Zika virus to Colorado), Travel drives pandemics (2015 World Cup brought Zika to Brazil, causing a pandemic that mostly affected pregnant women)
How can wildlife trade ead to emerging infectious diseases?
Consumption of wildlife meat can lead to emerging infectious diseases by bringing diverse species into contact and spreading many different diseases, Variable squirrels traded and were 46.4% positive for Leptospira spp. 2-3 squirrels = 83% chance of infection
How can invasive alien species lead to emerging infectious diseases?
Reservoirs: Australian brushtail possum – introduced to New Zealand – famous reservoir for bovine TB and spreading it to cattle, Grey squirrels introduced to UK bringing parapoxvirus, red squirrels highly susceptible, grey squirrels reservoirs
Vectors: Aedes mosquitoes, Originates from sub-Saharan Africa, Now found in temperate and tropical areas across the globe, Vector: Yellow fever, Dengue fever, Chikungunya & Zika
Pathogen: Yellow fever, introduced to many continents through slave trade from West Africa
Transmission dynamics: Burmese python
How can climate change lead to emerging infectious diseases?
Host parasite interactions: New England Moose (Ghost Moose): Populations declining, Impact of climate change on ticks allows them to breed and survive for longer on calves and White-tailed deer bringing brain worm with them northwards as they move to colder areas due to climate change, which caused co-infections with New England Moose ticks, lung worms, and bot flies
Thermal performance curve: Malaria, Thought warmer temperatures =
better for mosquitoes Temperature fluctuations affect life history traits &
parasite’s incubation period, Found that the peak temperature for malaria transmission (R0) is lower than previous estimates
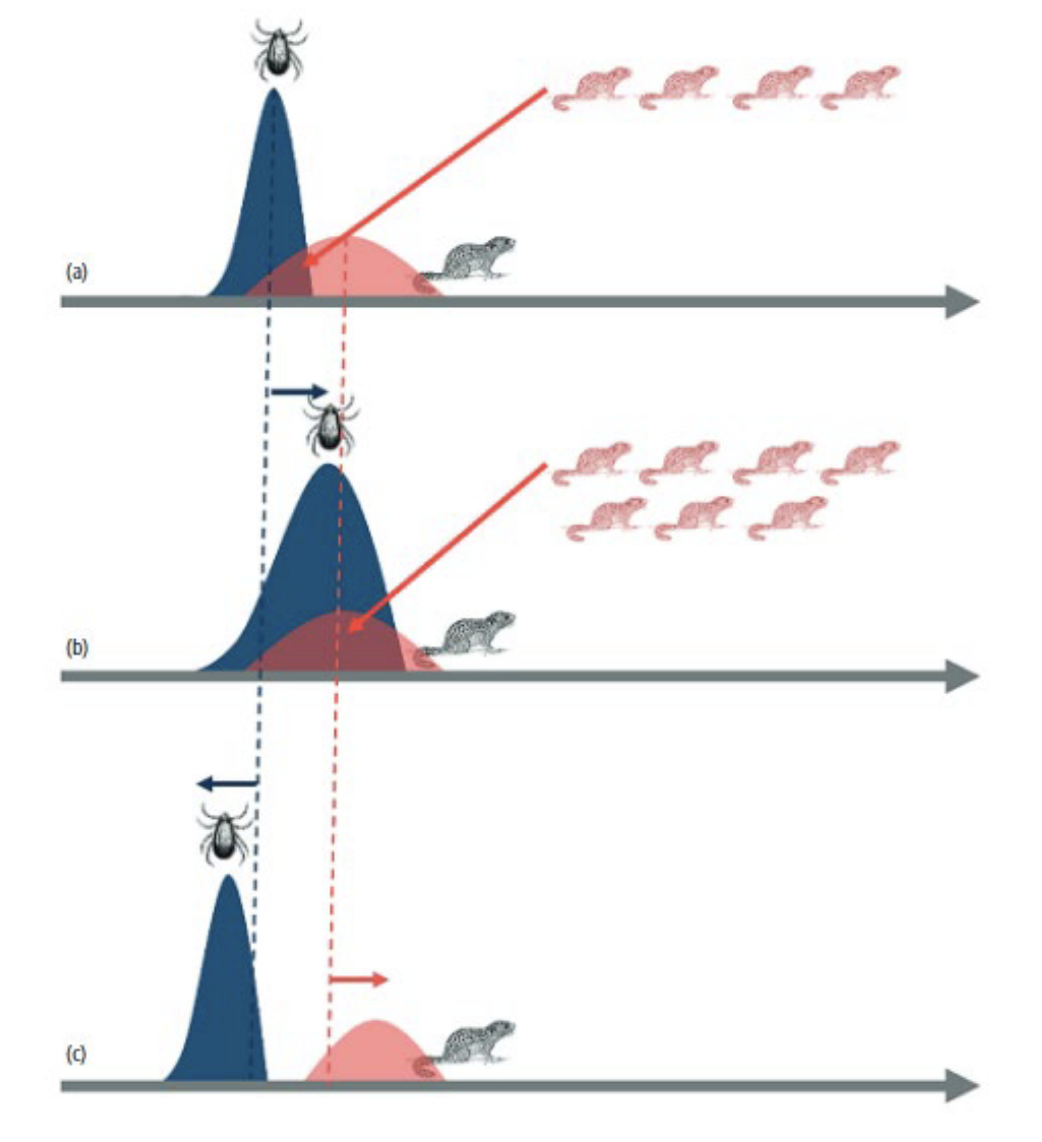
Explain this diagram
Disease transmission (red squirrels) occurs as a result of vector abundance
(blue) overlapping host exposure (orange)
Changes in vector phenology, i.e. a longer activity season, increases the
window of overlap with host animals, increasing the prevalence or
abundance of infection
In contrast, increased asynchrony in the phenology of vector and host disrupts
pathogen transmission
Explain the saiga antelope climate change example.
Saiga antelope are an endangered species that started experiencing a mass die off that killed 2/3 of their population in a few weeks. Die off was due to Pasteurellosis infection that increased due to unusually warm and humid conditions
What aspects of disease does climate change affect?
spatial distributions of hosts, vectors, and pathogens, but may just as often lead to declines or shifts rather than expansions
Explain the land-use change hendravirus example.
Hendra virus is spread by flying foxes, Clearing of trees = increasing numbers of bats moved into towns and cities, Started roosting in cities year-round, human-associated food resources include fruit trees planted in horse paddocks in more rural areas NEW INTERACTIONS = bats & horses
How is milk farming linked to the spread of COVID-19?
Mink were caged individually to protect fur quality, but kept at high densities= airborne virus could still spread, most likely introduced by infected farm workers
and stray cats
How did vampire bats lead to the spread of livestock rabies?
Changes in land use, such as deforestation and the introduction of livestock, have increased contact between bats, humans, and domestic animals, vampire bats prefer livestock over wildlife, livestock can be fed on up to 12 times a day, half a million livestock in Latin America died in on year
What is parasite fitness?
Refers to how well a parasite can survive, reproduce, and spread within a host population.
What are challenges in wildlife management in relation to threshold for invasion (NT)?
Sparse data makes NT hard to determine, Populations are spatially structured, not randomly mixed, Diseases often have multiple hosts or reservoirs, Environmental factors (season, climate, habitat) impact disease dynamics, Demographic stochasticity: Random birth/death events affect small populations. Thresholds are useful for theory, but not always reliable in practice
Why are wildlife diseases hard to manage?
● Diseases are often left unmanaged unless it threatens human health.
● Many diseases persist even below theoretical thresholds.
● Empirical limitations make accurate modeling difficult.
● Real-world complexities (latent periods, vectors, environmental transmission) blur simple thresholds.
What does the “do nothing” approach to wildlife disease control consist of?
● Disease is natural, part of ecosystem function - Natural processes are allowed to play out.
● May serve as compensatory mortality (e.g., balancing predation).
● Can be cost-effective, but may allow uncontrolled spread.
● Often used when:
○ Resources are limited.
○ Disease has minimal impact on humans or key species.
● Sometimes no intervention is chosen, especially when risks of action outweigh benefits.
What does the “ Reduce Disease in Reservoir Species” approach consist of?
● A reservoir is a host population where the disease persists and from which it can spread or a species that harbors the disease and can transmit it to others without being seriously affected itself.
● Eliminate or isolate reservoirs (e.g., culling or fencing).
● Strategies:
○ Treat or vaccinate reservoir populations.
○ Reduce density of infected individuals.
What is the Belgium rabies example in the “Reduce Disease in Reservoir Species” approach?
● Aerial vaccine bait campaigns (1989–1994).
● Resulted in ~80% eradication of rabies in the target area.
● Why it worked: Consistent effort, appropriate vaccine for the species.
What is the Rabies in the U.S. example in the “Reduce Disease in Reservoir Species” approach?
● Different reservoirs: Raccoons, skunks, bats.
● Current vaccines are ineffective in some species (e.g., raccoons, fatal in some skunks).
● Bat reservoirs present unique challenges due to mobility and ecology.
What is the Sylvatic Plague example in the “Reduce Disease in Reservoir Species” approach?
● Ongoing issue in the U.S. affecting prairie dogs and associated species, like the endanngered black-footed ferrets (that prey on priarie dogs).
● Vaccine efforts are in development and deployment.
What is the Bovine Tuberculosis (TB) in the U.K. example in the “Reduce Disease in Reservoir Species” approach?
● Linked to badgers as reservoir species.
● Culling efforts (1975–1996):
○ Initial success within the culling zone, but TB rebounded.
○ Randomized trial (1998):
■ Compared reactive culling, proactive culling, and no culling.
■ Unexpected outcome: TB increased in reactively culled areas compared to control areas
■ Culling changed the behavior of the badgers – they simply moved out of the culling zone to the fringe/edge areas – TB increased in those areas even though it technically decreased within the culling zone
■ Long-term study showed no real benefit of culling badgers for controlling Tb transmission in UK
What are some main problems with culling?
o May cause vacuum effects – new animals move in.
o Can change social structure and increase contact rates.
o May fail to reduce disease or even make it worse.
What methods are being used to limit reproduction in reservoirs?
o Reduces population density and contact rates.
o Field challenges:
§ Surgical sterilization (e.g., gonadectomy) impractical.
o Promising method: GonaCon™
§ A contraceptive vaccine targeting reproductive hormones.
§ Reduces reproductive behavior and disease transmission.
Not Effective: PZP (Zona Pellucida) vaccines—may increase mating behavior.
What does the “Reducing Disease in Target Species” approach entail?
● Targeting the species you want to protect (rather than reservoir).
● May involve vaccination, habitat management, or altering behavior.
Consists of three approaches: vaccination, treatment, or culling
Explain the Oral vaccination of wild dogs of the “Reducing Disease in Target Species” approach. Why is this approach problematic?
● Targeted vaccination aims to increase resistance in at-risk populations.
● Issues:
○ Rarely tested with proper scientific controls.
○ Hard to justify leaving animals unvaccinated for experimentation.
○ Often reactive “crisis management” during disease outbreaks.
○ No consistent evidence that vaccination improves survival in wild populations (see examples in table)
Explain why the treatment aspect of the “Reducing Disease in Target Species” approach.
● Often resource-intensive and stressful for animals.
● Effectiveness frequently untested.
● Needs long-term commitment.
What are some examples of the treatment aspect of the “Reducing Disease in Target Species” approach.
○ Fox tapeworm (Echinococcus multilocularis): Baiting with anthelminthics reduced disease in Japan.
○ Koalas (Chlamydia): Eye and bladder symptoms; requires medical treatment.
○ Wombats (Sarcoptic mange): High mortality due to poor diet, low metabolism.
What is the goal of culling in the “Reducing Disease in Target Species” approach.
● Goal: Remove superspreaders or reduce population density.
● Situational effectiveness:
○ Only works if disease spread is density-dependent.
○ Must be careful not to reduce population below viable levels.
What are some examples of culling in the “Reducing Disease in Target Species” approach.
○ Tasmanian Devil Facial Tumor Disease: Not effective due to frequency-dependent transmission.
○ Chronic Wasting Disease: Focus on large, adult males; resistance from hunters may hinder effectiveness.
What are some ways to go about the “Reducing Transmission Between Reservoir and Target” approach?
● Barrier creation, habitat separation, or synchronized management across species.
● Fencing: Physical barriers prevent interspecies transmission.
○ Used for Foot-and-mouth disease virus in South Africa to separate the buffalo (reservoirs) from cattle that live outside the Kruger National Park.
○ Problem is that elephants are respsonible for the majority of fence-breaks.
○ Expensive and difficult to control elephants which makes controlling FMDV difficult.
● Fencing:
○ Separates bighorn sheep from domestic sheep in California.
○ Important way to control disease spread
What disease control method works the best?
No one-size-fits-all solution.
● Effectiveness depends on:
○ Disease type and transmission dynamics
○ Target and reservoir species biology
○ Practical, ethical, and ecological constraints
● Often a combination of strategies works best
What is the disease profile for Chagas disease?
Infectious agent type: parasitic protozoan
Host species: definitive host is the triatomine bug (kissing bug)
Reservoir species: most wildlife species and humans
Vectors: Triatomine Bugs
Transmission mode: bite from the vector, vertical transmission, blood
transfusion or organ donations, and contaminated food
How infection occurs/spillover: Primary vectors biting humans in endemic areas
Case fatality rate: low
Treatments/vaccines: Benznidazole and Nifurtimox (only effective during certain stages)
What is the disease profile of Sea Star Wasting Disease?
○ Infectious agent type: not fully known but most likely a bacteria
○ Host species: various sea star species
○ Reservoir species (if known): sea stars
○ Vectors (if vector-borne): N/A
○ Transmission mode: direct and indirect contact with pathogen
○ How infection occurs or how spillover occurs (if zoonotic): Not zoonotic
○ Case fatality rates: Depends on species, can be very high
○ Treatments / vaccines: In development
What is the disease profile for Bovine TB?
○ Infectious agent type: Bacteria
○ Host species: Cattle
○ Reservoir species (if known): White-tail deer, buffaloes, badgers, possums, wild boar
○ Vectors (if vector-borne): None
○ Transmission mode: Ingestion of food, Respiratory secretions- saliva, mucus, etc.
○ How infection occurs or how spillover occurs (if zoonotic): Consumption of raw milk is most common zoonotic spillover cause
○ Case fatality rates: low
○ Treatments / vaccines: BCG vaccine, Antibiotics, Culling, X-rays, Genome sequencing
What is the hantavirus disease profile?
○ Infectious agent type: ssRNA
○ Host species: Deer mouse
○ Reservoir species (if known): many rodent species
○ Vectors (if vector-borne): None
○ Transmission mode: exposure to infected rodent saliva, urine, or feces
○ How infection occurs or how spillover occurs (if zoonotic): mice to humans through bites
○ Case fatality rates: 30-50%
○ Treatments / vaccines: No vaccine, at the onset of symptoms with known risk to
exposure seek supportive care, Avoid infected rodents where possible
What is the disease profile for HIV?
○ Infectious agent type: Virus
○ Host species: Humans
○ Reservoir species (if known): Humans
○ Vectors (if vector-borne): None
○ Transmission mode: Direct contact with infected bodily fluids
○ How infection occurs or how spillover occurs (if zoonotic): Not zoonotic but SIV led to development of HIV
○ Case fatality rates: Used to be very high, has declined overtime
○ Treatments / vaccines: No vaccine, PrEP for pre-exposure, ART for post-exposure
What is the disease profile for Valley Fever?
○ Infectious agent type: Infectious fungi
○ Host species: Humans, pets, livestock, some wildlife, zoo animals
○ Reservoir species (if known): N/A
○ Vectors (if vector-borne): N/A
○ Transmission mode: inhalation of the spores once the soil it was growing in is disturbed
○ How infection occurs or how spillover occurs (if zoonotic): Not zoonotic
○ Case fatality rates: 1.14%
○ Treatments / vaccines: No vaccine, Nikkomycin Z (NikZ) has been successful with dogs
What is the disease profile of Newcastle Disease?
○ Infectious agent type: Alulavirus
○ Host species: Birds, humans, pests/insects
○ Reservoir species (if known): Birds
○ Vectors (if vector-borne): N/A
○ Transmission mode: direct contact of contaminated droppings, respiratory secretions, or inhaling particles in the air
○ How infection occurs or how spillover occurs (if zoonotic): Spreads to lab workers/others in close contact with infected birds
○ Case fatality rates: Depends on species, can be low or high
○ Treatments / vaccines: Vaccine for birds
What is the disease profile for Chronic Wasting Disease?
○ Infectious agent type: Transmissible Spongiform Encephalopathy (prion)
○ Host species: Cervids
○ Reservoir species (if known): Cervids
○ Vectors (if vector-borne): None
○ Transmission mode: Direct contact with body fluids of infected individuals, indirect contact through environmental contamination
○ How infection occurs or how spillover occurs (if zoonotic): Not zoonotic
○ Case fatality rates: 100%
○ Treatments / vaccines: None