Unit 3 Chemistry Review
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Formula mass/ Molecular Mass
The sum of the atomic masses of the atoms in a molecule
The Mole
a unit of measurement pertaining to the number of molecules in a sample.
Avogardo’s Number
The number of items in a mole which is approximately 6.022 × 10²³
Molar Mass
The mass of one mole of a substance, usually expressed in grams per mole (g/mol), which is numerically equal to the molecular or formula mass of the substance. uses amu as a conversion factor.
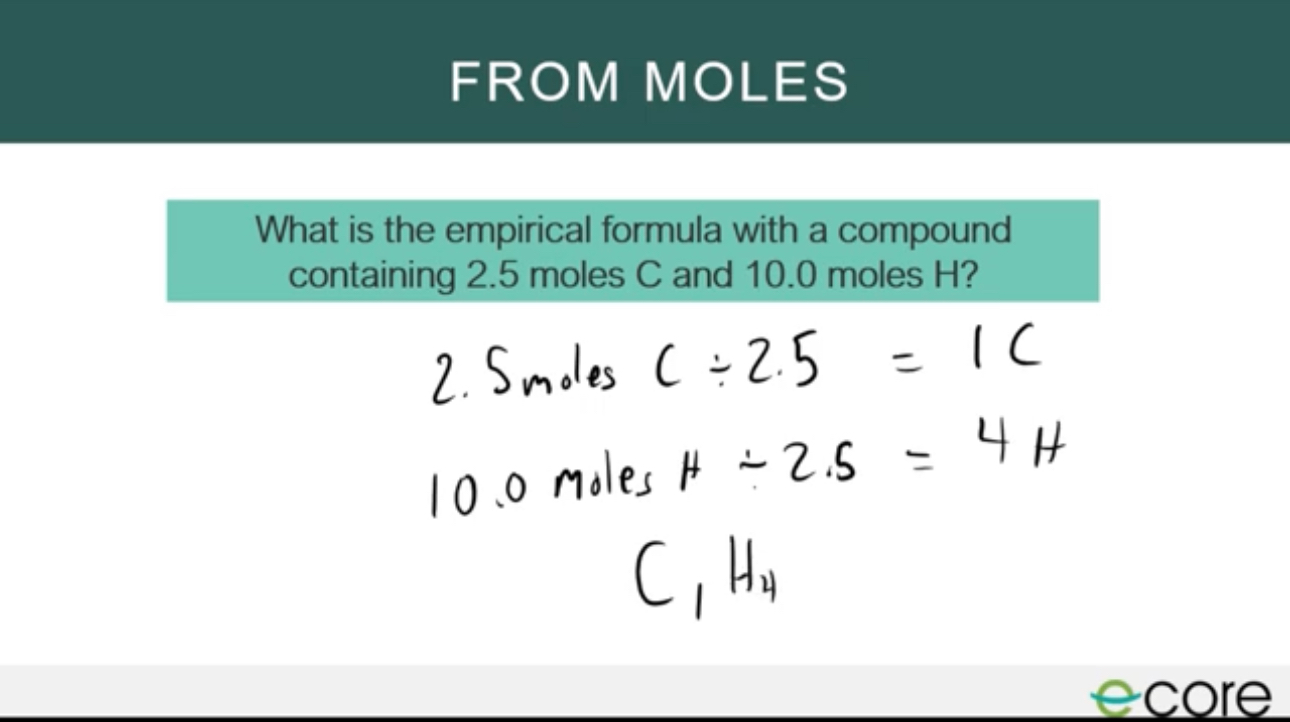
Empirical Formula from moles
divide each amount of moles for an element by the smallest number of moles to obtain the simplest ratio of the elements in a compound.
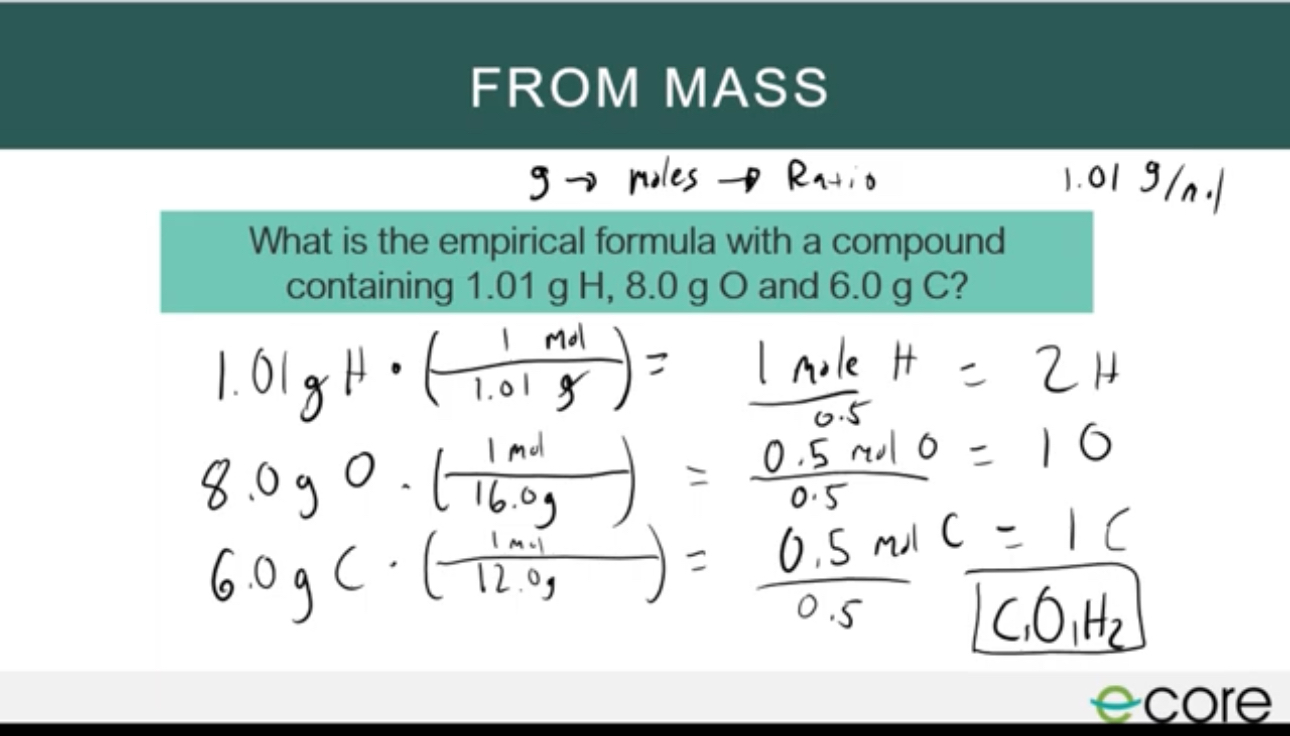
Empirical formula from mass
mass x 1 mol/ molar mass of the substance and then divide your answer by the smallest number
Empirical Formula from Percent By Mass
Convert percent to grams, assume 100 g sample, and then find moles of each element before dividing by the smallest number of moles.
Finding Molecular formulas from Empirical Formula
divide molar mass by empirical formula mass and you multiply your answer by the subscripts of the empirical formula to get the formula of the compound.
A solution
Contains one component with a concentration that is significantly greater than all other components( called a solvent)
A solution in which water is a solvent is called an ——- ——-
Aqueous solution
A Solute
A component of a solution that is typically present at a much lower concentration than the solvent