Organic Chemistry II Exam 1: Chapter 15 "Benzene and Aromatic Compounds"
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Benzene
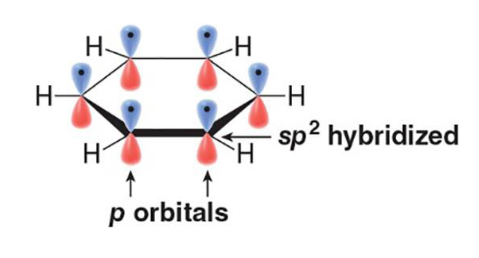
The simplest aromatic hydrocarbon. Very unsaturated yet does not readily undergo addition reactions. Reacts with Bromine only in the presence of a Lewis acid in substitution. Planar with equal bond lengths. Sp2 hybridized, with p orbitals extending above and below the plane of the molecule. Extremely stable.
Kekule
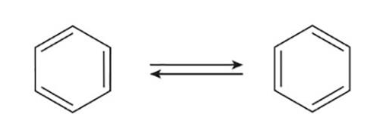
What is the structure that states benzene is a rapidly equilibrating mixture of two compounds, each containing a six membered ring with three alternating bonds?
Resonance Hybrid
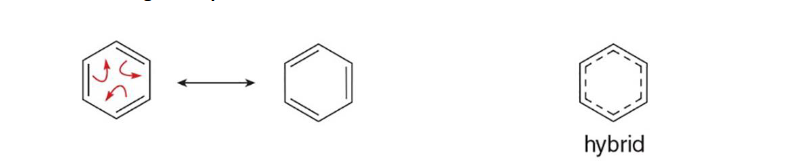
True benzene is represented by what?
electrophiles
Benzene is electron rich and reacts with strong (nucleophiles/electrophiles)
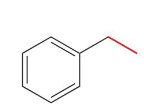
ethylbenzene
Name this molecule
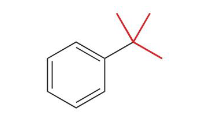
tert-butyl-benzene
Name this molecule
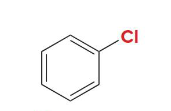
Chlorobenzene
Name this molecule
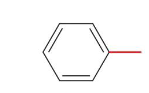
Toluene (methylbenzene)
Name this molecule
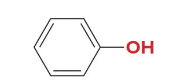
phenol (hydroxybenzene)
Name this molecule
Aniline (aminobenzene)
Name this molecule
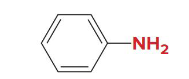
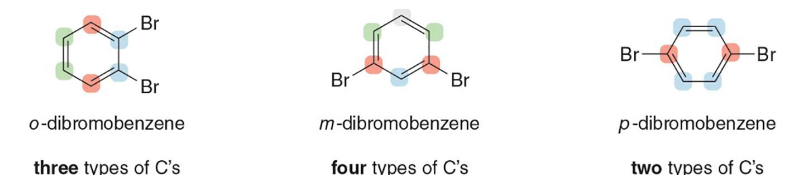
ortho
Is this dibromobenzene in ortho, meta, or para position
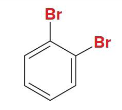
meta
Is this dibromobenzene in ortho, meta, or para position
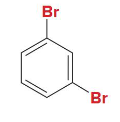
para
Is this dibromobenzene in ortho, meta, or para position
o-bromochlorobenzene
Name this molecule
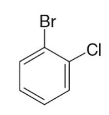
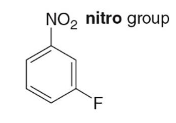
m-fluoronitrobenzene
Name this molecule
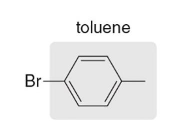
p-bromotoulene
Name this molecule
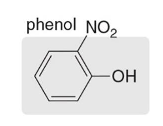
o-nitrophenol
Name this molecule
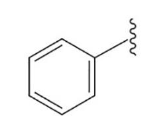
phenyl
This group is created by removing one hydrogen from benzene. C6H5—. A substituent of benzene (aryl group)
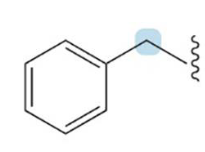
benzyl
A group that is a substituent of benzene (aryl group). C6H5CH2
C NMR
The number of signals on this spectrum indicates the number of the types of carbons in the target molecule. The types of carbons are determined by their immediate surroundings, and that similarity to other carbons in the molecule
Aromatic
(Aromatic/Antiaromatic) Compounds
1) Must be cyclic
2) Must be planar
3) Must be completely conjugated
4) Must satisfy Huckel’s Rule: 4n+2 pi electrons
5) All carbons must be sp2 hybridized
Ex: benzene
Antiaromatic
(Aromatic/Antiaromatic) Compounds
1) Must be cyclic
2) Must be planar
3) Must be completely conjugated
4) Have 4n pi electrons
Ex: Cyclobutadiene

Find the answer in notes
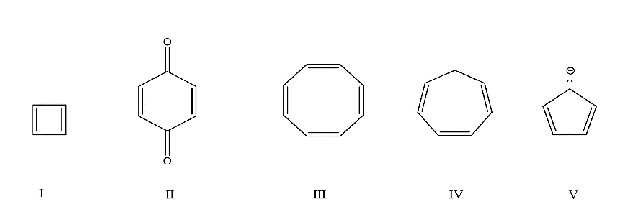
Which of the following molecules is aromatic?
Heterocycles
These benzene like rings contain oxygen, nitrogen, or sulfur and can be aromatic
Aromatic (6 electrons)
Is this molecule aromatic, antiaromatic, or nonaromatic?
Antiaromatic (4 electrons)
Is this molecule aromatic, antiaromatic, or nonaromatic?
nonaromatic (5 electrons)
Is this molecule aromatic, antiaromatic, or nonaromatic?
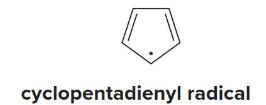
Aromatic
Is this molecule aromatic, antiaromatic, or nonaromatic?
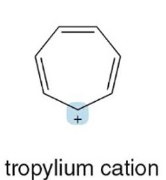
Molecular Orbital Theory
This theory, (not valence bond theory), describes bonds as the mathematical combination of atomic orbitals that form a new set of orbitals called molecular orbitals. EX: when two p orbitals combine, two molecular orbits should form, and that bonding is either constructive or destructive
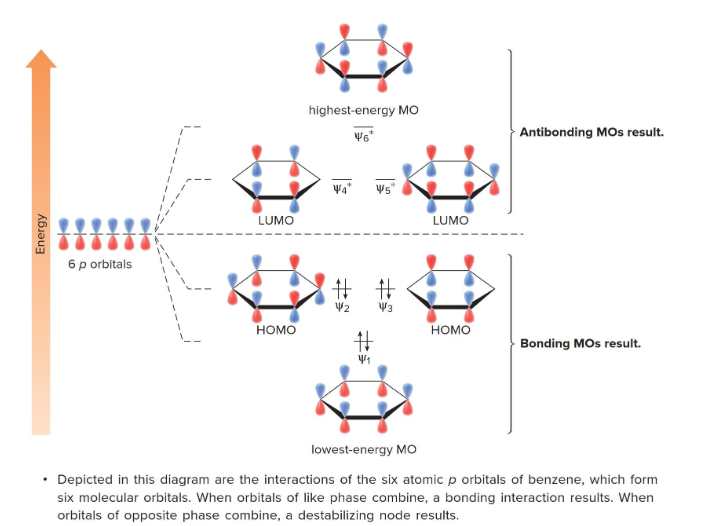
Benzene
This graph shows the molecular orbits for which molecule?
2, 6, 10, 14
List the magic numbers of aromaticity