Portage CHEM 103 Module 3
1/85
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
86 Terms
Solid Ionic compound
- Solid under normal conditions
- Crystalline structure and tends to be rigid and brittle
- High melting point
- Extremely high boiling point
- Not electrically conductive because the forces that hold cations and ions together are really strong and hard to break so cations and anions cannot flow therefore heat and electricity cannot flow
- When molten it can conduct electricity
- When dissolved in water it can conduct electricity as the ions break apart in the water and get encapsulated by the water molecules so the charged particles are able to move around freely in water and solution therefore when electricity is applied to the solution, ions carry the electricity through the solution
Ionic Bonding
Neutral atoms and their associated ions have very different physical and chemical properties
Binary
Composed of just two elements
Binary Ionic Compound
- Metal cation(s) + Nonmetal anion(s)
- All resulting substances must be electrically neutral
Electronic Structures of Cations
Forms from the loss of electrons from valence shell
Isoelectronic
- When two different elements have the same electron configuration
- The same # of electrons in the same arrangement
Group 1 Akali Metals
1 valence electron
Form +1 charge
Group 2 Alkaline Earth Metals
2 valence electrons
Form +2 charge
Group 13 Boron group
3 valence electrons
Form +3 charge
Post Transition Metals
- Group 13-17
- Can form a charge by subtracting 10 from group #
- Ex: group 13 = +3 charge
Variable Charges
- Can be formed by transition, post transition and inner transition elements by partial loss of valence electrons
- Because they have electrons in their 'd' and/or 'f' orbitals as well as their 's' orbitals of the highest principle energy level
- They have electrons they can lose to form different charges
Electronic Structure of an Anion
- Valence shell is only determined by the s and p orbitals
- Aiming to reach the electron configuration of the next noble gas
Covalent Bond
- Attractive force between the nuclei of a molecule's atoms and pairs of electrons between the atoms
- Formed between two atoms when they both have similar tendencies to attract electrons to themselves
- Formed by nonmetallic elements
Covalent Compound
- Held together by covalent bonds
- Composed of molecules formed by atoms of two or more different elements
Physical Properties of Molecular Compounds
Often gases; liquid or gas at room temp, solids are "soft"
- Low boiling point liquids and low melting point solids because they are held together by relatively weak forces compared to the forces in ionic compounds
- Mostly insoluble in water
- Most are poor conductors of electricity in any state
- Water is polar and will dissolve anything that has a charge like ions or also polar
- Molecular compounds are electrically neutral so it can't conduct electricity because ions must be able to move in the substance and molecular compounds are not ions
The Formation of Covalent Bonds
Covalent bonds formed between nonmetal elements
Ex: H2
Intermolecular Distance for H-H Bond
- Where two hydrogen atoms share their electrons and have the least amount of energy
- Most stable
- 74 picometers/ 7.24 x 10^-9J
Bond Length
Determined by the distance at which the lowest potential energy is achieved
Breaking Chemical Bonds
- Endothermic process
- Energy is required
- Same energy required to break a bond is the same amount to make a bond
Forming Chemical Bonds
- Exothermic process
- Energy is released
- Same energy required to break a bond is the same amount to make a bond
Pure Covalent Bond
- Electrons are shared equally between atoms
- Identical atoms share identically bc they have the exact same attraction for the shared pair of electrons
- Electron density (probability of finding electron) around either atom is identical
Polar Covalent Bond
- Electrons are not equally shared between atoms
- Bonding electrons are more attracted to one atom than the other; two different elements/ atoms attracting electrons
- Results in a partial positive charge on one atom and a partial charge on the other causing the electron to spend more time closer to one of the atoms than the other
- Shift in electron density or the distribution of where the electrons may be located in the atom
- Results in separation of charge
Electronegativity
- Measure tendency of an atom to attract electrons (electron density) towards itself
- The more strongly an atom attracts shared electrons, the larger its electronegativity therefore the atom that has a stronger attraction has a higher electronegativity
- The more electronegative atom will bear a partial negative charge because that's where the electrons spend most of their time
- Greater difference in electronegativity means a more polarized electron distribution
- Value calculated by Linus Pauling ranging from 0 to 4.0 (arbitrary scale) with the least electronegativity being 0 and the most being 4.0
- Has to do with an electron's attraction to the nucleus of an atom
Electronegativity Left to Right
Increases
Electronegativity Down the Column
Decreases
Electronegativity Compared to Electron Affinity
- Dimensionless quantity that is calculated, not measured
- Describes how tightly an atom attracts electrons in a bond
- Bonding situations only
Electron Affinity Compared to Electronegativity
- Measurable physical quantity
- Energy released or absorbed when an isolated gas-phase atom gains an electron
- Creation of an anion
Determining Bond Polarity
- Find the absolute value of the difference in electronegativity of two bonded atoms
- If the two elements are close on the periodic table they most likely have very similar electronegativities, if far very different electronegativities
Pure Covalent Electronegativity
< 0.4
Polar Covalent Electronegativity
0.4 to 1.8
Ionic Electronegativity
> 1.8
Easiest/Quickest determination of bond type
Look at the identity of atoms in the bond:
Two nonmetals generally covalent
Metal + nonmetal generally ionic
Special Ionic Compound
Polyatomic ions are held together by covalent bonds but form ionic compounds by combining with tons of opposite charge
Binary Compound Naming Rule
Name of the cation (name of the metal) followed by name of anion (name of the nonmetal, change the ending to "-ide")
Polyatomic Ion Naming Rule
Name of the cation (name of the metal or polyatomic) followed by name of anion (name of the nonmetal, change the ending to "-ide" or polyatomic)
Do NOT change endings of polyatomic ions
Compound Containing a Metal Ion with a Variable Charge
- Cation + anion ("-ide" or polyatomic)
- If metal can form variable charges, specific charge with a Roman numeral in parentheses after metal's name
- You can tell what Roman numeral to use by determining the charge of the anion by looking at the ratio in the compound e.g FeCl2 = Fe^2- Cl^- Iron (II) chloride but FeCl3 = Fe^3- Cl^- Iron (III) chloride
Hydrates
- Compounds that contain water molecules as integral components of their crystal
- Name derived from anhydrous compound name, adding term to indicate how many water molecules are present per ionic formula unit
Ex: Copper (II) sulfate pentahydrate
Anhydrous
Ionic compound without water
Hydrate Naming Rule
Cation (metal w/ roman number if necessary) + anion (nonmetal "-ide" or polyatomic) then add appropriate numerical Greek prefix to "-hydrate"
Covalent/Molecular Compounds composed of 2 elements Naming Rule
- Nonmetallic elements can form multiple compounds with combinations of the same elements e.g. CO & CO2
- Name must reflect combination, cannot be figured out based on charges the way ionic compounds are
- Name the more metallic element, adding a Greek prefix if two or more atoms are present
- Name the more nonmetallic element adding a Greek prefix and change the ending to "-ide"
- Elements more to the left or bottom of the periodic table are more metallic
Acids
- Special and important
- Compounds that release hydrogen ions (H+) when dissolved in water
Binary Acid
Made of hydrogen and one other nonmetallic element
Binary Acid Naming Rule
- Add prefix "hydro-"
- Name nonmetallic element, changing the ending to "-ic"
- "acid" is added as a second word
Oxyacid
Contains hydrogen, oxygen and at least one other element (usually a polyatomic, oxygen containing ion) and are bonded to impart acidic properties to the compound
Oxyacid Naming Rule
- Start with the root name of the anion
- Replace "-ate" with "-ic" or "-ite" with "-ous"
- Add acid
Lewis Symbol
- Used to describe the valence electron configurations of atoms and monatomic ions, consists of an elemental symbol surrounded by one dot for each of its valence electrons
- Can be used to illustrate the formation of cations or anions from atoms
- Can also be used to show the transfer of electrons during the formation of an ionic compound
Lewis Structure
Describes the bonding in molecules and polyatomic ions
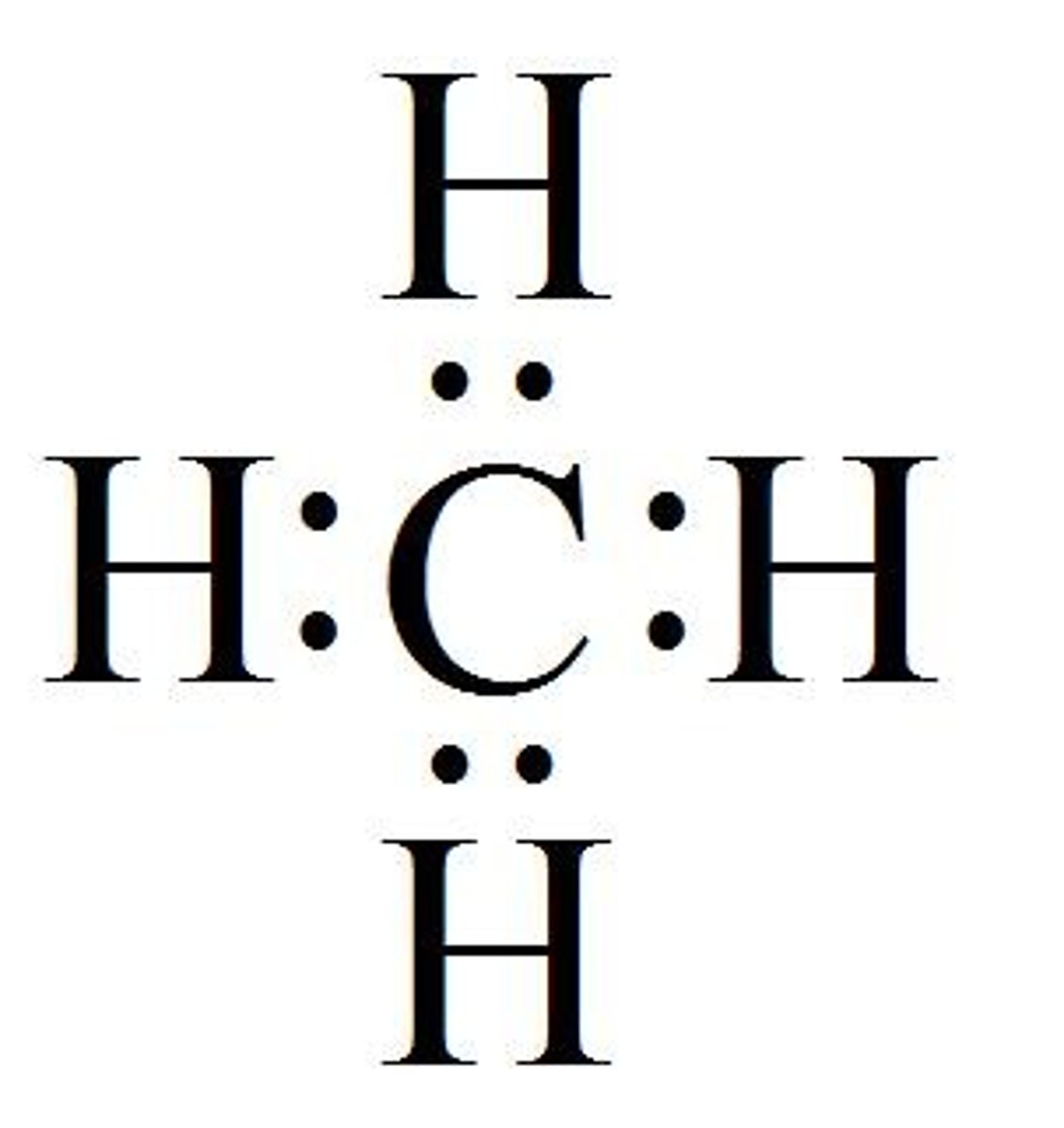
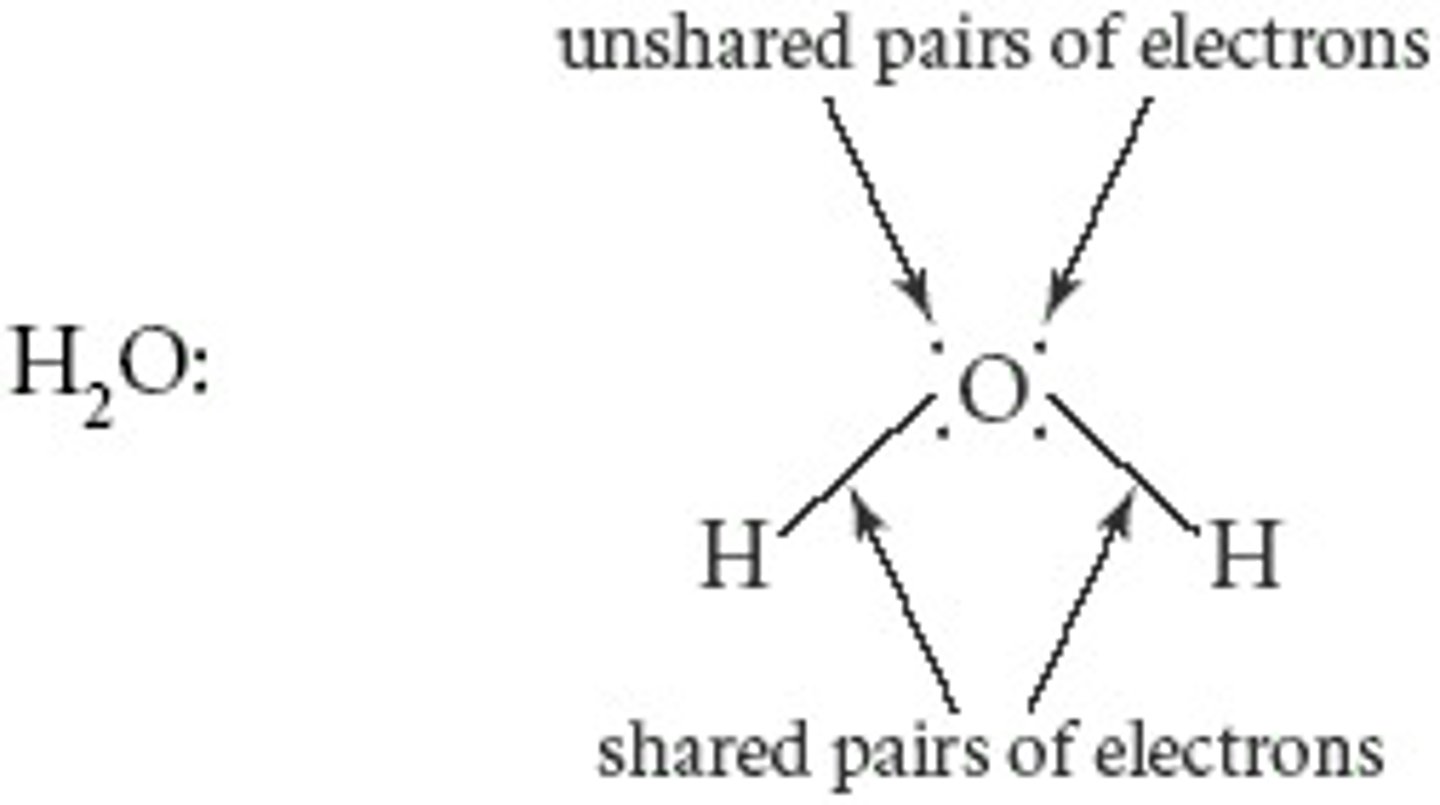
Shared Pair
- Electrons shared between two nuclei in a bond
- Shown as stacked dots or lines between atoms/element symbols
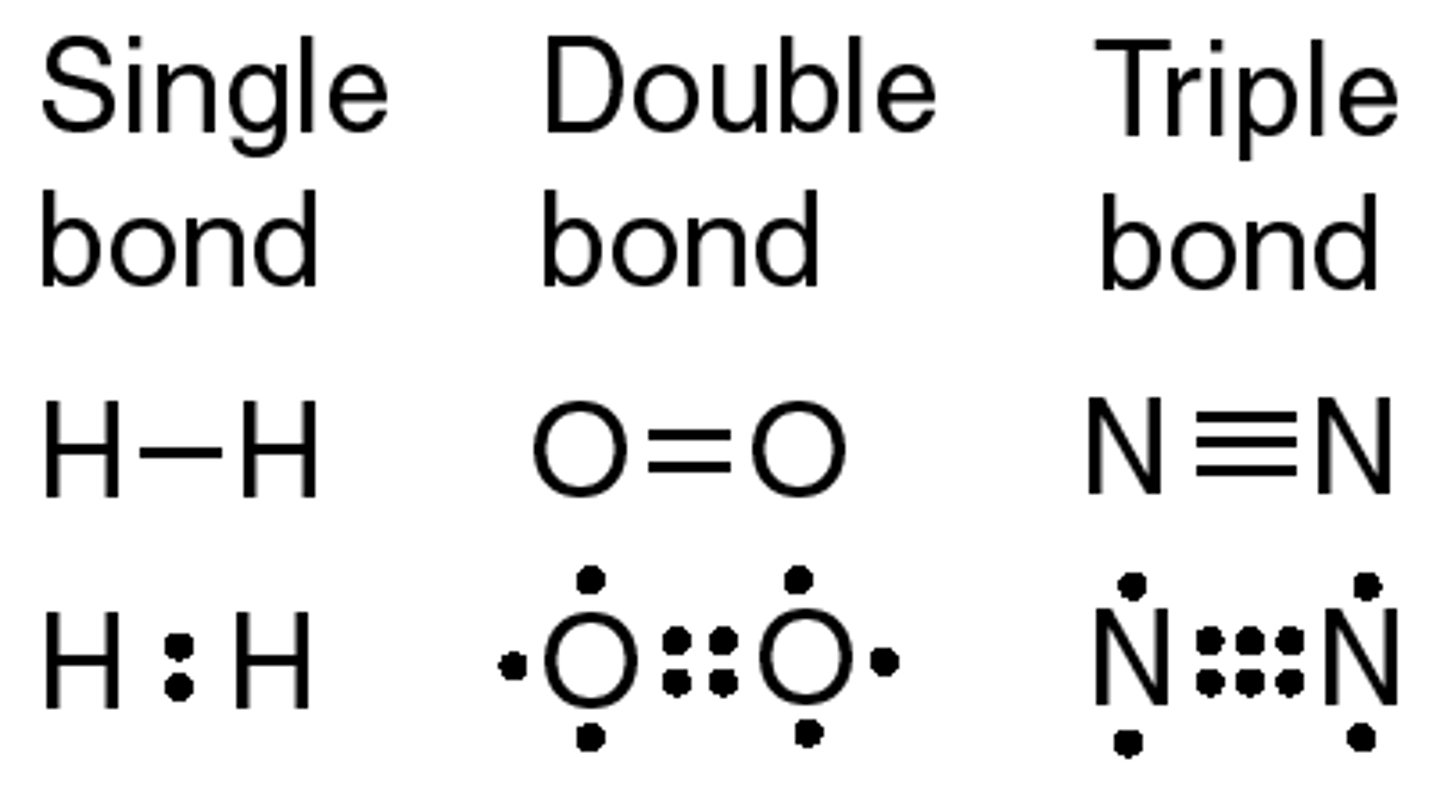
Lone Pair
Electrons that are not used in bonding
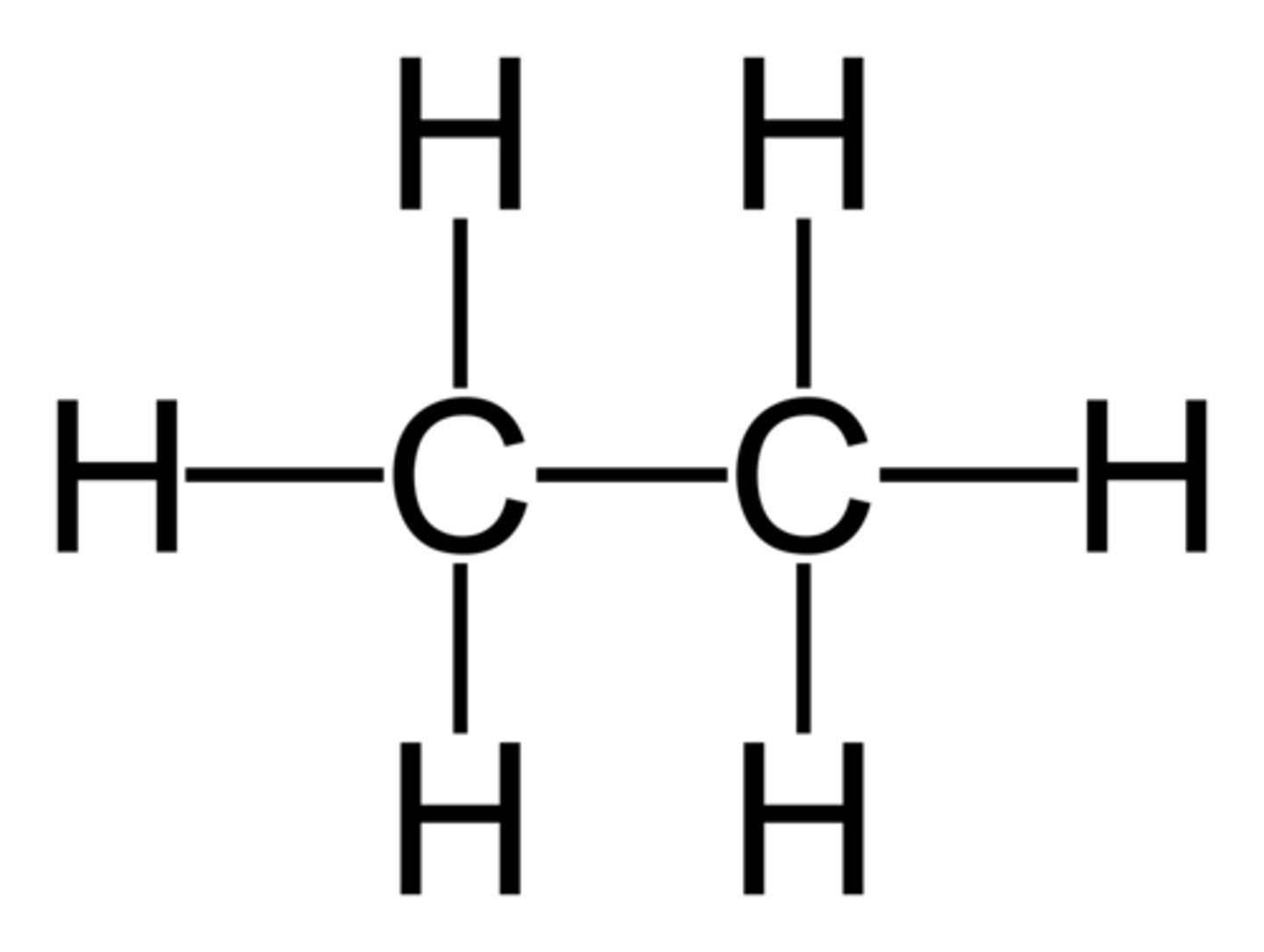
Single Bond
Single pair of electrons shared between two atoms
Octet Rule
- Tendency of main group atoms to form enough bonds to obtain eight valence electrons
- If one pair of electrons doesn't satisfy octet rule atoms may share more than one pair of electrons
- Exception: Hydrogen only needs two electrons to fill its valence shell
Double Bond
Covalent bond where two pairs of electrons are shared between atoms
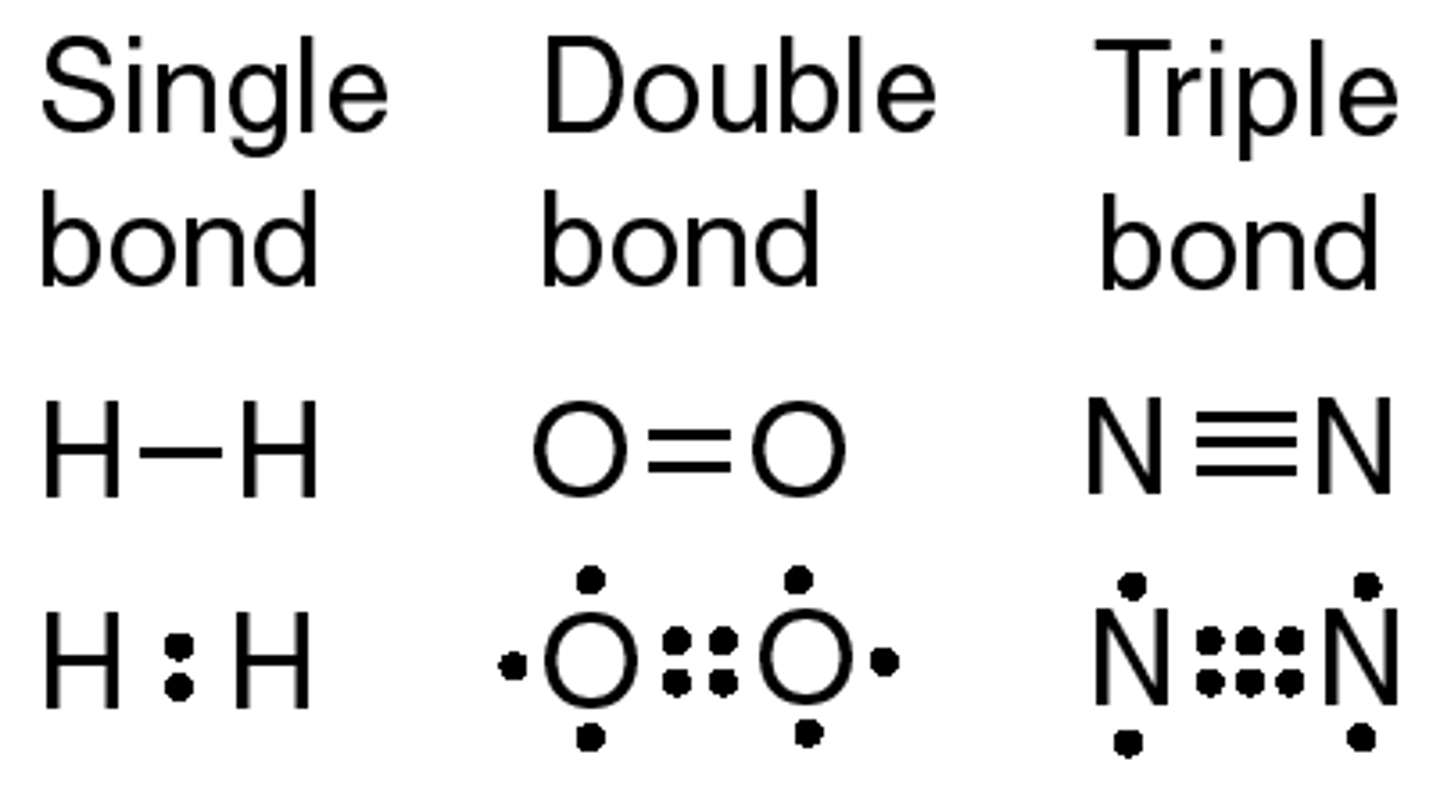
Triple Bond
Covalent bond where three pairs of electrons are shared between atoms
Drawing Complicate Molecules 101
1. Determine the total # of electrons; for cations subtract one electron for each positive charge, for anions add one electron for each negative charge
2. Draw skeleton structure; Least EN element in the center, surround with the other atoms. Connect each atom to the central atom to the central atom with a single bond
3. Distribute the remaining electrons to the outermost atoms as lone pairs to complete the octet
4. Place all remaining electrons on the central atom
5. Rearrange the electrons of the outer atoms to make multiple bonds with the central atom in order to obtain octets wherever possible
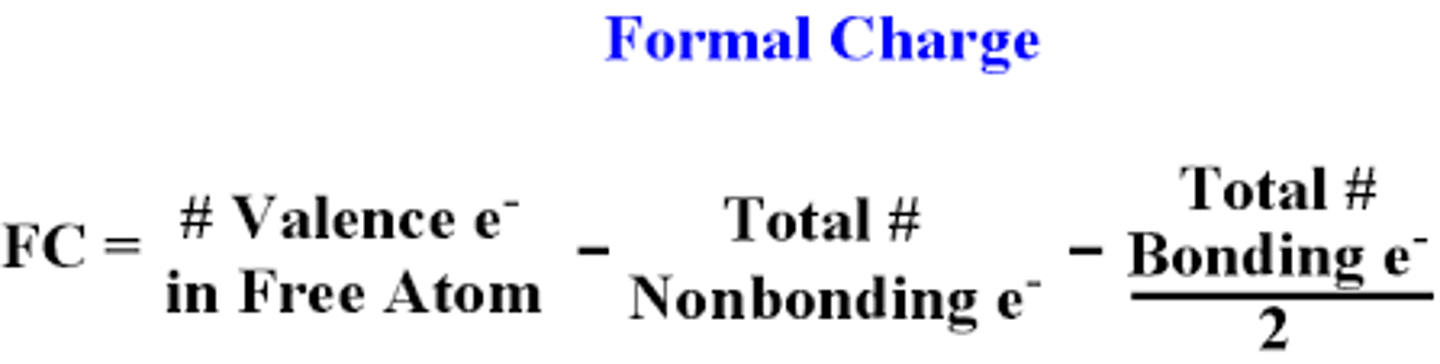
Formal Charge
- Hypothetical charge of an atom in a molecule if the electrons in the bonds were redistributed evenly between the atoms
- Sum of formal charges of all atoms in a molecule must be zero
- Sum of the formal charges in an ion should be equal to the charge of the ion
Molecular Structure
- Arrangement of atoms in a molecule or ion
- Use formal charges to decide the most likely molecular structure
- Preferable for all atoms to have a formal charge of zero
- If charge of zero isn't possible, it's preferable that atoms have a formal charge as close to zero as possible
- Preferable for adjacent atoms to have formal charges of zero or of the opposite sign
- Preferable for the more electronegative atoms to have any negative formal charges
Resonance
- One Lewis structure is insufficient to describe the bonding in a molecule and the average of multiple structures is observed
Resonance Forms
Two or more Lewis structures that have the same arrangement of atoms but different arrangements of electrons
Resonance Hybrid
Average of the resonance forms shown by the individual Lewis structures, the actual electronic structure
Lewis Structure
Two dimensional representation of three-dimensional molecules showing the electron location and connectivity of atoms
Bond Angles
Angles between any two bonds that include a common atom usually measured in degrees
Bond Distance
- Distance between the nuclei of two bonded atoms along the straight line joining the nuclei
- Measured in Ångstroms; 1Å = 10^-10m or 1pm = 10^-12m, 100pm in 1Å
VSEPR Theory
- Valence-shell electron-pair repulsion theory
- Predicts the molecular structure, including approximate bond angles around a central atom, of a molecule from an examination of the # of bonds and lone electron pairs in its Lewis structure
- Electron pairs are arranged to minimize repulsion/maximize distance; bonding pairs, lone pairs
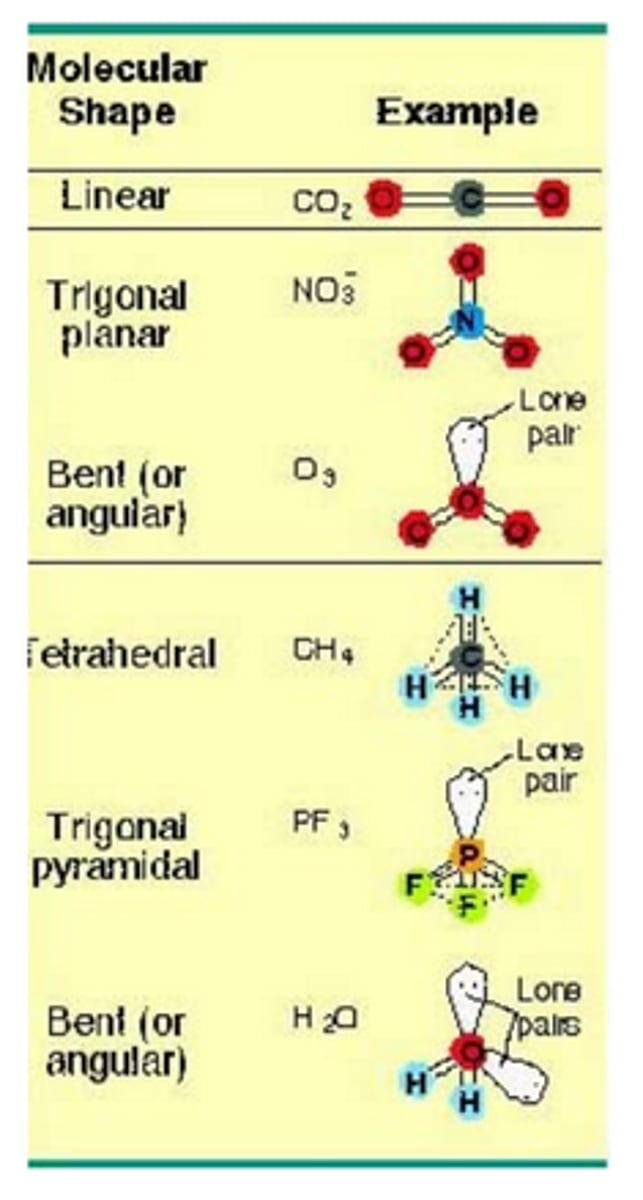
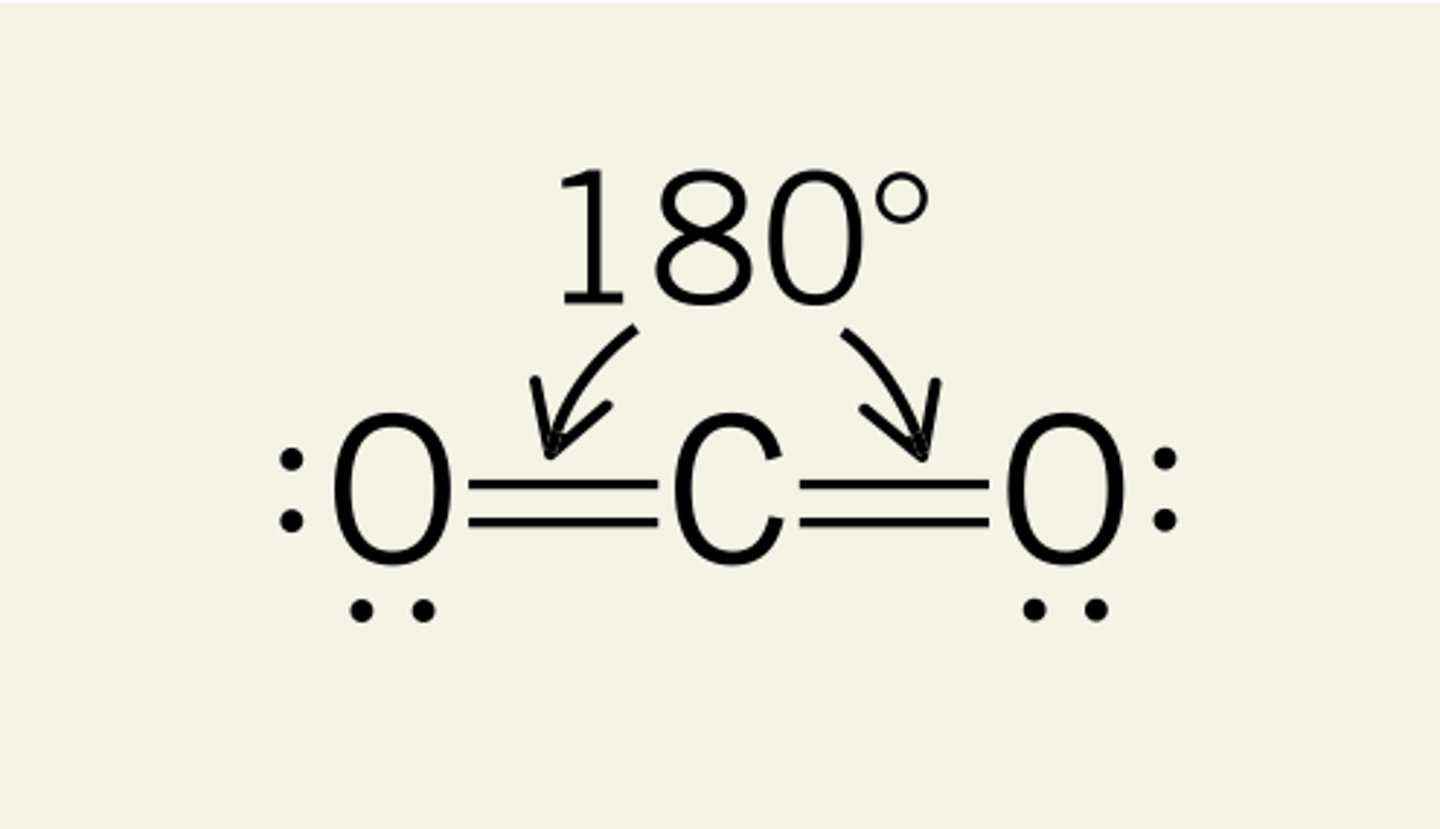
Linear
Two regions of electron density around a central atom
180 deg
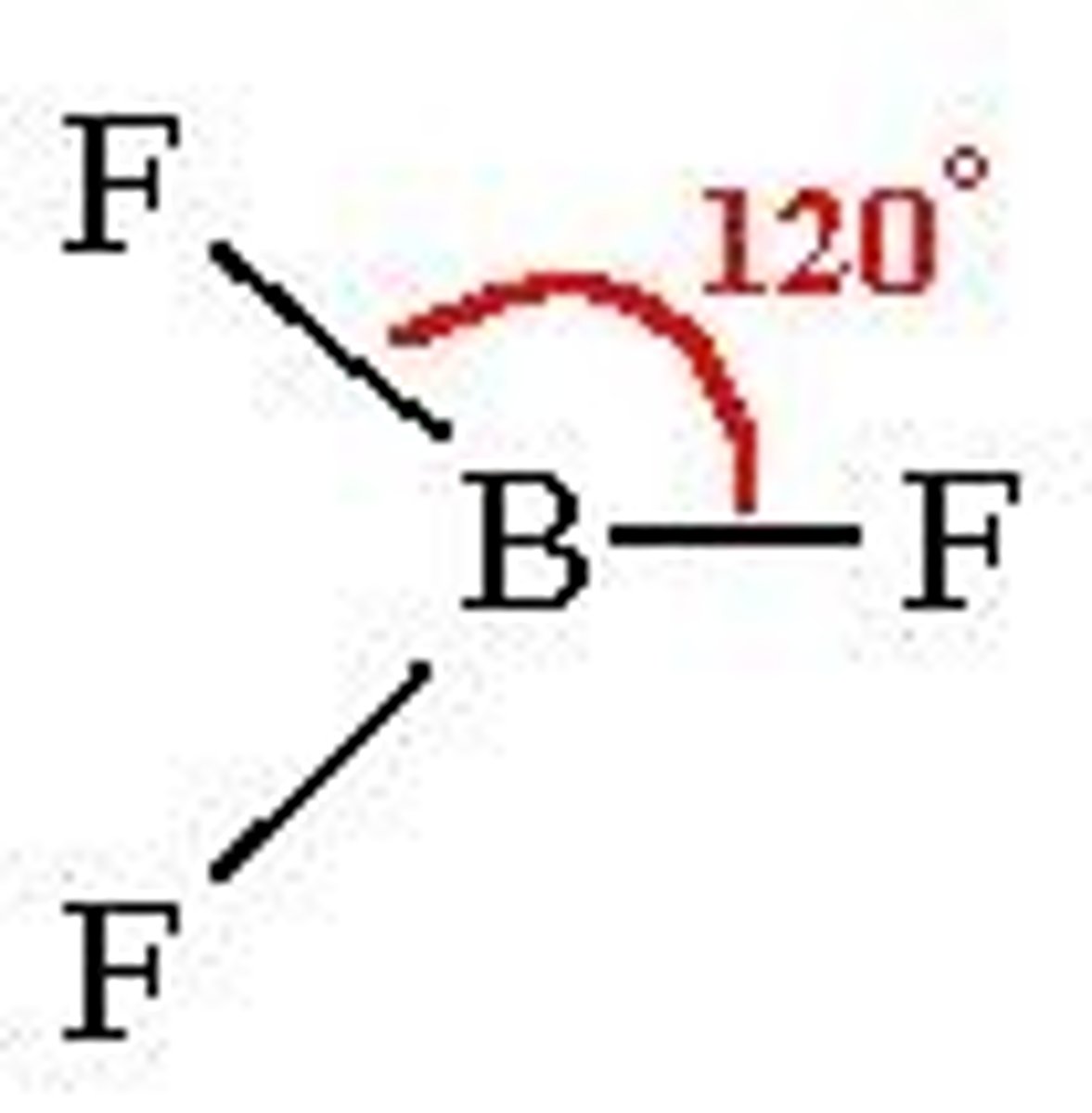
Trigonal Planar
Three regions of electron density around a central atom
120 deg
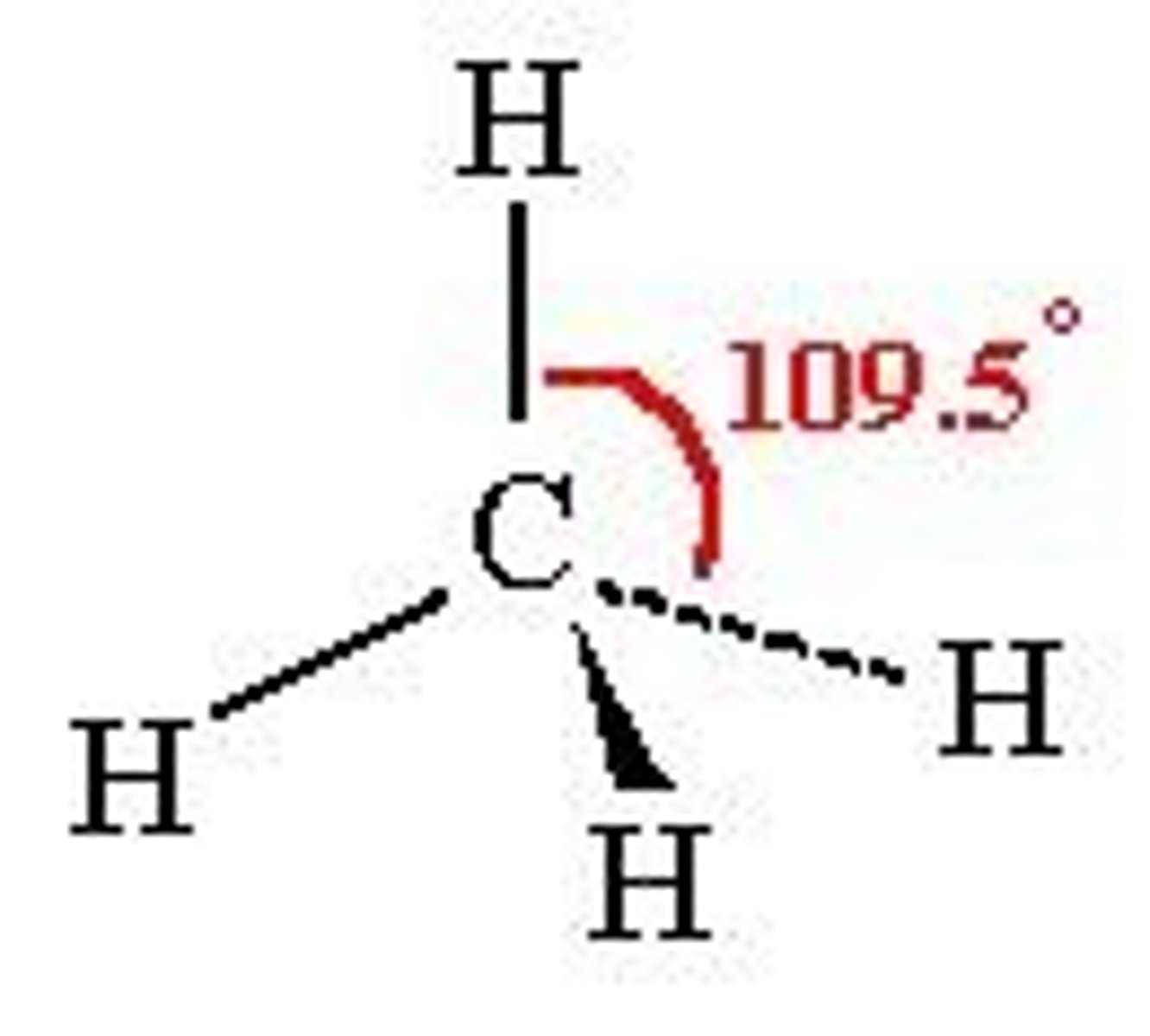
Tetrahedral
Four regions of electron density around a central atom and 3D
109.5 deg
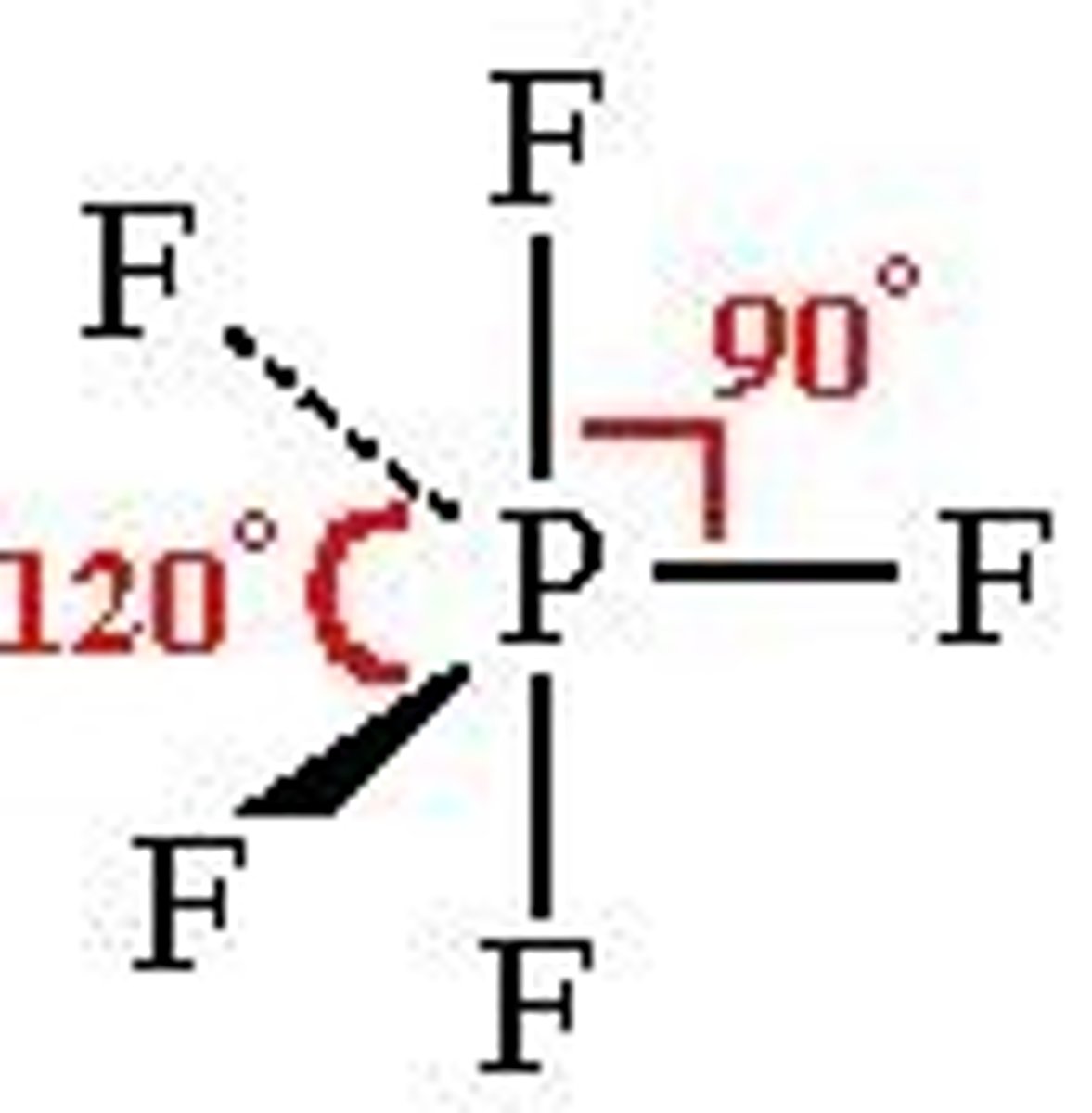
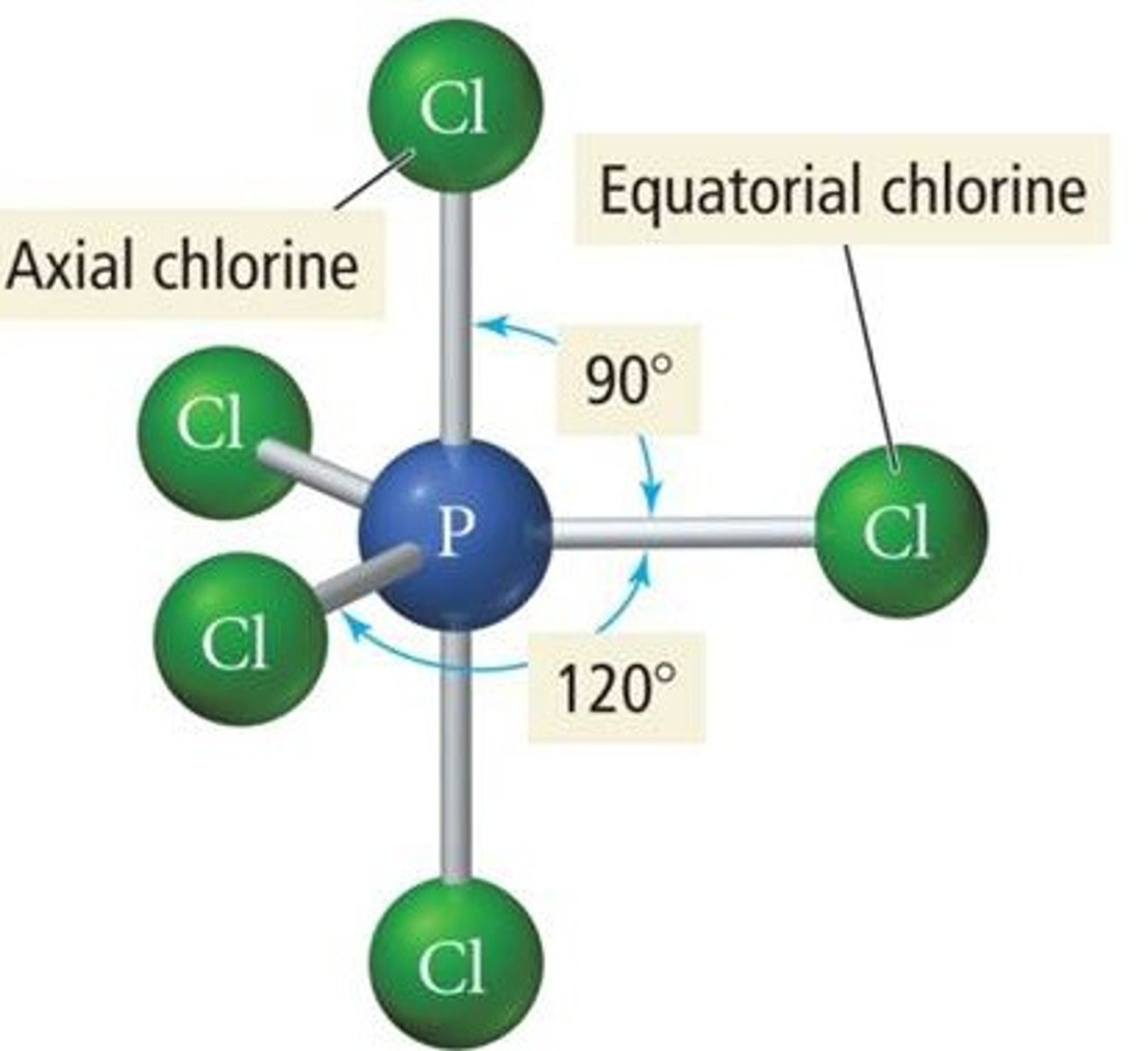
Trigonal Bipyramidal
- Five regions of electron density around a central atom and 3D
- Only one where every position is not equivalent
- some atoms are 90 deg and some are 120 deg
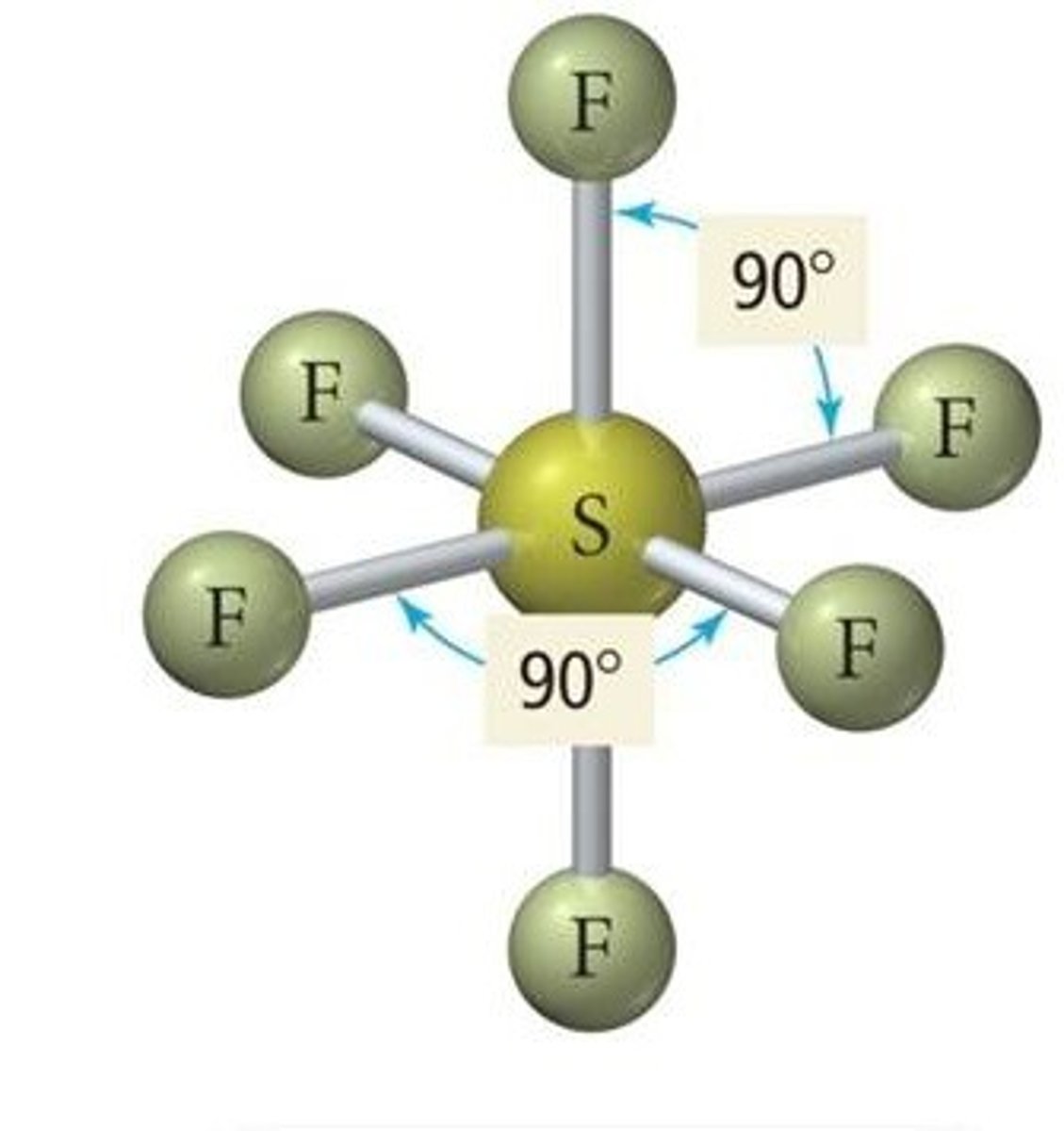
Octahedral
Six regions of electron density around a central atom and 3D
90 deg
Electron Geometry
- Arrangement around a central atom including all regions where electrons are located
- Bonds and Lone pairs
Molecular Geometry
- Arrangement around a central atom including only the placement of atoms
- Bonds only
- Lone pairs are present but not considered for the shape name
- Derived from electron geometry
- Lone pairs occupy larger regions of space than bonding electrons, they push atoms away and make angles smaller
Order of Greatest Electron Repulsion to least
Lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair
Order of Sizes from Largest to Smallest
Lone pair > triple bond > double bond > single bond
Axial Position
- Special feature of trigonal bypyramidal molecular geometry
- Location at which there is another atom at a 180 degree angle and three atoms at a 90 degree angle - "axis"
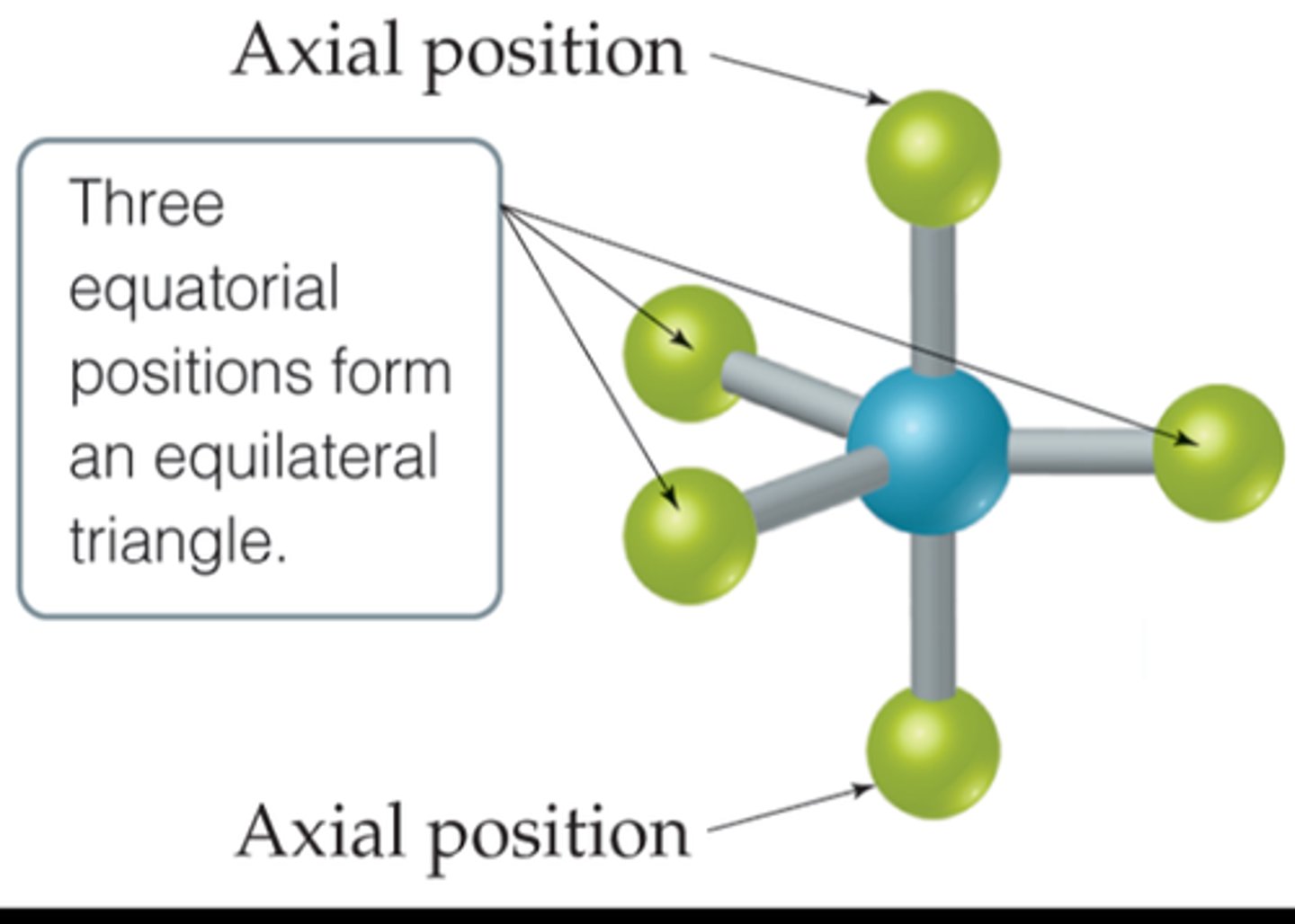
Equatorial Position
- Another special feature of trigonal by pyramidal molecular geometry
- Location at which there are two other atoms at 120 degree angles and two atoms at 90 degree angles - "equator"
- Lone pairs fo équatorial before axial
Predicting Electron & Molecular Geometry
1. Write the Lewis structure
2. Count the # of regions of electron density around the central atom
3. Identify the electron geometry
4.Use the # of lone pairs to determine the molecular geometry
5. The most likely structure will minimize repulsions
Molecular Geometry for Multicentre Molecules
- Larger molecules do not have a single central atom but they are connected by a chain of interior atoms that each possess a "local" geometry
- Consider each atom in the chain individually as a central atom
Polar Covalent Bonds
Connects two atoms with differing EN leaving the less EN atom with a partial positive and the more EN atom with a partial negative charge
Bond Dipole Moment
- Separation of charge in a bond
- Can be represented using vectors
Vector
- Quantity with both a direction and magnitude
- Arrow points along the bond toward the atom with the partial negative charge
- Plus sign at the end of arrow is above the atom with the partial positive charge
- Length of arrow is proportional to the charge separation
- A whole molecule can have a separation of charge depending on its molecular structure and the polarity of each of its bonds
Polar Molecule/ Dipole
- Molecule with a separation charge
- Uneven charge distribution
Dipole Moment
- Description of the net charge separation in the molecule as a whole
- Determined by adding all the bond moments in 3D space, taking the molecular structure into account
Nonpolar
Molecule with no separation of charge; even charge distribution
When Bond Dipole Moment is the Same As Dipole Moment
- Homonuclear diatomic molecules
- Heteronuclear diatomic molecules
When Bond Dipole Moment is NOT the Same As Dipole Moment
Polyatomic molecules
Determining the polarity of polyatomic molecules
- Consider molecular geometry (VSEPR)
- Consider bond dipole moments
To be polar, a molecule must?
- Contain at least one polar covalent bond
- Have a molecular structure where the sum of the bond vectors does not cancel