Biochemistry Section C
1/138
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
139 Terms
What factors define life?
Metabolism, growth and reproduction
What is the fundamental unit of life? Why?
The cell, simplest unit capable of life, all organisms are made of cells.
List the Hierarchy of life
molecules, organelles, cells, tissues, organ, organism, population, community, ecosystem, biosphere
What makes up 50% of cell composition?
Proteins
Most enzymes are what?
proteins (some are RNA)
Where are proteins synthesized?
ribosomes
What are enzymes?
Proteins that speed up chemical reactions
How do enzymes speed up chemical reactions?
They act as a catalyst and lower activation energy therefore increasing reaction rates.
What does catalytic activity depend on?
the integrity of the native protein conformation. Structure affects function!
Explain enzyme-substrate specificity
The active site of an enzyme is very specific to its substrates resulting in enzymes being able to catalyze only certain reactions.
What determines enzyme-substrate specificity?
1. Binding energy of enzyme. The weak interactions that bind the substrate and enzyme are specific and only react with specific molecules.
2. Arrangement of atoms in active site
Why are biocatalysts better than inorganic catalysts?
Greater specificity (avoid side products), milder reaction conditions (compliments body conditions), higher reaction rates (useful timeframe), and capacity for regulation (control biological pathways).
Name 3 inorganic catalysts
Iron, Vanadium Oxide, and Manganese Oxide
Name two organic catalysts (biocatalyst)
Digestive and metabolic
What are some characteristics of enzymes?
Highly efficient, highly specific, lower activation of energy, and reduced time to reach equilibrium
What do enzymes NOT change?
Equilibrium constant, equilibrium concentrations, or free energy change of reaction
What is the equilibrium constant for substrates and products?
Keq= [P]/[S]
Explain the equilibrium constant for substrates and products
When movement of products and substrate back in forth in reversible reaction eventually reach equilibrium.
Measures if we have more product or substrate.
What is the ground state?
the starting point for either the forward or the reverse reaction
What is the transition state?
the point at which decay to substrate or product are equally likely
What is the standard free energy change?
free energy change of a reaction. Can be spontaneous (releases energy) or nonspontaneous (requires energy)
What is activation energy?
difference between the ground state energy level and transition state energy level.
How does an enzyme decrease activation energy?
By decreasing transition state energy
What are the seven major classes of enzymes?
oxidoreductases, transferases, hydrolases, lyases, isomerases, ligases, and translocases
What reaction do oxidoreductases catalyze?
oxidation-reduction reactions
Transfer of electrons
What reaction do transferases catalyze?
group transfer reactions
What reaction do hydrolases catalyze?
hydrolysis reactions
transfer of functional groups to water
What reaction do lyases catalyze?
Formation of double bonds by removal of groups or addition of groups to double bonds
What reaction do isomerases catalyze?
Transfer of groups within molecules to yield isometric forms
What reaction do ligases catalyze?
Bond formation coupled with ATP hydrolysis
What reaction do translocases catalyze?
Movement of molecules or ions across membranes or their seperation from membranes.
What are simple enzymes?
enzymes that consist of protein alone
What are complex enzymes?
enzymes that consist of a protein plus a cofactor
What is a prosthetic group?
when a cofactor is tightly or covalently bound to the enzyme
What is a coenzyme?
when a cofactor is non-covalently bound to the enzyme
What is a holoenzyme?
Complete complex of protein and cofactors
What is an apoenzyme?
protein part of holoenzyme
Vitamin can act as what type of cofactor?
coenzyme, non-covalently bound
Metal ions can act as what type of cofactor?
prosthetic group, covalently bound
What is one example of clinical diagnosis regarding enzymes?
CPK, LDH, HBDH are enzymes found in the heart, when someone has a heart attack, they are released into the blood. A higher concentration of these enzymes in a blood test indicates heart attack.
What is one example of treatment of diseases regarding enzymes?
Aspirin can act as an enzyme inhibitor for cyclooxyrgenase, an enzyme that catalyzes a reaction that produces pain.
How does enzyme specificity affect the number of reactions it can catalyze?
Enzymes will only catalyze a singular reaction or sometimes a few closely related reactions.
Where do enzyme reaction occur? What binds here?
the active site; substrates
How do ligand binding sites and active sites differ?
a reaction occurs at an active site- conversion of a substrate to a product
What are some active site properties?
Takes up small part of enzyme, formed by groups from different parts of AA sequence, and it is a cleft/cervice.
How are substrates bound to active site?
Through multiple weak, reversible interactions
Why is the lock and key model wrong?
An enzyme completely complimentary to a substrate is a poor enzyme.
Why is the induced fit model correct?
Enzymes that favor the transition state rather than substrate induce conformation change of substrate to reach the transition state.
Who proposed that enzymes bind to transition state best?
Linus Pauling
How does binding energy contribute to catalytic power?
enzymes bind most tightly to the transition state and use binding energy to lower activation energy
What is binding energy?
The energy produced when a substrate non covalently binds to an enzyme.
What is binding energy's role?
The sum of the unfavorable activation energy and the favorable binding energy results in a lower activation energy. Overcomes barriers to a reaction.
What is a characteristic of binding energy?
It is sometimes used to hold two substrates in the optimal orientation for reaction
What are the 4 barriers to a reaction?
Entropy of the molecules, solvation shell of hydrogen bonded water that surround biomolecules, distortion of substrates, and the need for proper alignment of catalytic functional groups.
How does binding energy overcome the entropy barrier?
constrains substrates in proper orientation in order to react with eacother
How does binding energy overcome solvation shell barrier?
Creates weak interactions with substrate. This replaces hydrogen between the substrate and water that normally inhibit the reaction.
How does binding energy overcome distortion of substrate barrier?
A substrate may need to undergo unfavorable change when bound in order to react. Binding energy helps compensate for change.
How does binding energy overcome proper alignment of functional groups barrier?
Brings specific functional groups on enzyme to proper position to catalyze the reaction
What is an acid-base catalyst?
Protons are transferred between an enzyme and a substrate.
What is a specific acid-base? General?
only uses H+ or OH- ions present in water; uses acids or bases other than water
How do acid-base catalyst aid in product formation?
Charged intermediates in a reaction without a catalyst can easily breakdown into the reactants. However, an acid-base catalyst can transfer the protons to stabilize the reaction.
Visual image of acid-base catalyst
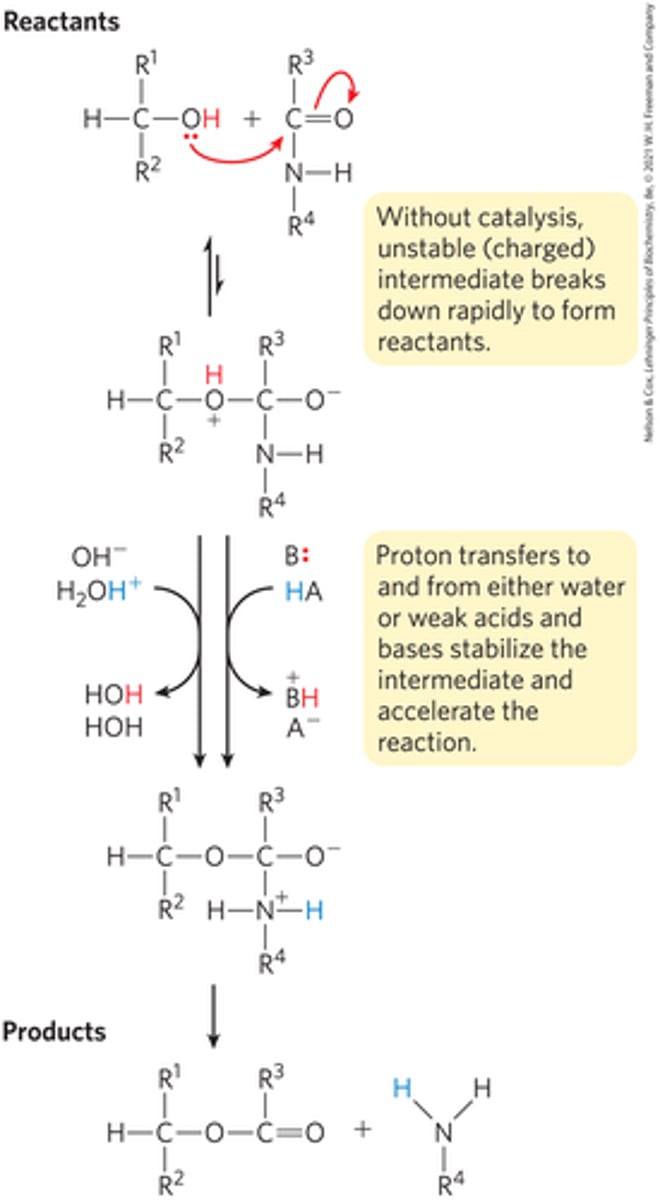
Amino acids can act as what kind of acid-base catalyst?
General. Proton donor at low pH and acceptor at high pH
What is a covalent catalyst?
transient covalent bond forms between the enzyme and substrate.
When do covalent catalyst occur?
Only when it lowers the activation energy of the reaction. Must be faster than uncatalyzed reaction.
What is a metal ion catalyst?
Help orient the substrate for a reaction, and decrease entropy with stabilizing weak bonds.
What type of reaction do metal ion catalysts mediate?
Oxidation-reduction reactions by reversible changes in the metals oxidation state.
Loss of electrons is oxidation and gaining electrons is reduction.
What combination of catalysts does chymotrypsin use?
Acid-base and covalent catalysts.
What is a protease? What enzyme displays this property?
An enzyme that catalyzes the hydrolytic cleavage of peptide bonds (breaks peptide bond by adding water); chymotrypsin
What site on the peptide does chymotrypsin cut?
cleaves the peptide bond adjacent to aromatic amino acids
What type of amino acids in peptides display aromatic rings?
Phenylalanine, Tyrosine, and Tryptophan
What is the catalytic triad?
The active site has His57, Ser195, and Asp102. These form a hydrogen-bonded constellation called catalytic triad
What is a scissile bond?
The bond on peptide that is to be cleaved.
Chymotrypsin Reaction Step One
The substrate binds, the side chain of residue adjacent to peptide bonds nestles in a hydrophobic pocket in the enzyme
Chymotrypsin Reaction Step Two
His57 and Ser195 generate a nucleophile by removing a proton from the Ser195 hydroxyl group. Ser195 hydroxyl group can now mount a nucleophilic attack on the C=O carbon of the polypeptide to give a tetrahedral intermediate with a negative carboxyl oxygen.
Chymotrypsin Reaction Step Three
This will cause a conformational change in the enzyme's active site and allow the negative carboxyl oxygen of the scissile peptide bond to move deeper into the active site. This is known as an oxyanion hole
Explain the oxyanion hole
This configuration, in which a negatively charged carboxyl oxygen is hydrogen bonded to two N-H groups
Chymotrypsin Reaction Step Four
Instability of negative charge on substrate carbonyl oxygen leads to collapse of tetrahedral intermediate which displaces bond between carbon and amino group of peptide. This causes peptide to break
Chymotrypsin Reaction Step Five
An incoming water molecules acts as a general acid-base catalyst. Acts as a nucleophile on ester link and recreates the tetrahedral intermediate on carbonyl group.
Chymotrypsin Reaction Step Six
The collapse of the tetrahedral intermediate forms the second product, a carboxylate anion group. This displaces Ser195 from the substrate.
Chymotrypsin Reaction Step Seven
Dissociation of the second product from active site regenerates the free enzyme.
How does Chymotrypsin lower the activation energy?
The oxyanion pocket stabilizes the tetrahedral intermediate and thereby lowers the activation energy for the reaction.
What is enzyme kinetics?
the study of the rates of which compounds react in enzyme-catalyzed reactions
What is the rate of enzymatic reactions affected by?
Enzyme, substrate, effectors, and temperature.
How do you define the rate of an enzyme?
expressed as products formed per unit of time or substrates consumed per unit of time.
What is denaturation? When does it occur?
The destruction of a protein; past 55 degrees C
What is initial velocity? When is it measured?
the rate of a reaction at the very beginning when the substrate concentration is highest; before more than ~ 10% of the substrate has been converted to product
What is maximum velocity? (Vmax)
the highest rate at which an enzyme can catalyze a reaction
When does maximum velocity occur?
occurs at high substrate concentration when the enzyme is saturated, when it is entirely in the ES form and there is no free enzyme
The value of the Vmax indicates what about the enzyme?
The Bigger the Vmax, the more powerful the enzyme
What is steady state?
When the rate of synthesis is equal to its rate of degradation
What is kcat?
the turnover number of the enzyme, i.e., number of substrate molecules catalyzed per enzyme molecule per unit time at saturation
What is Km?
the concentration of substrate which permits the enzyme to achieve half Vmax
How is Km related to enzyme affinity?
The lower the Km, the stronger the enzyme affinity. Requires less substrate to reach 1/2 max velocity
How do the concentrations of substrate and enzyme differ?
Substrate is at a much higher concentration than enzyme so we can assume the amount of substrates bound to an enzyme is small
What is the M-M equation of initial rate?
Vmax[S]/Km+[S]
Why is it difficult to assess the Vmax?
difficult to assess accurately from the direct plot of V vs [S].
What is a better way to find Vmax?
plot 1/V vs. 1/[S], called Lineweaver-Burk (or double reciprocal) plot. the inverse of the reaction rate against the inverse of the substrate concentration
What does the Lineweaver-Burk plot do?
linearizes the M-M kinetics data. The intercept of Y axis is 1/Vmax, the intercept of X axis is - 1/Km, and the slope of the line is Km/Vmax.
What is enzyme efficiency limited by?
kcat/Km