Unit 3 Chemistry
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
chemical change
A change in matter that produces one or more new substances
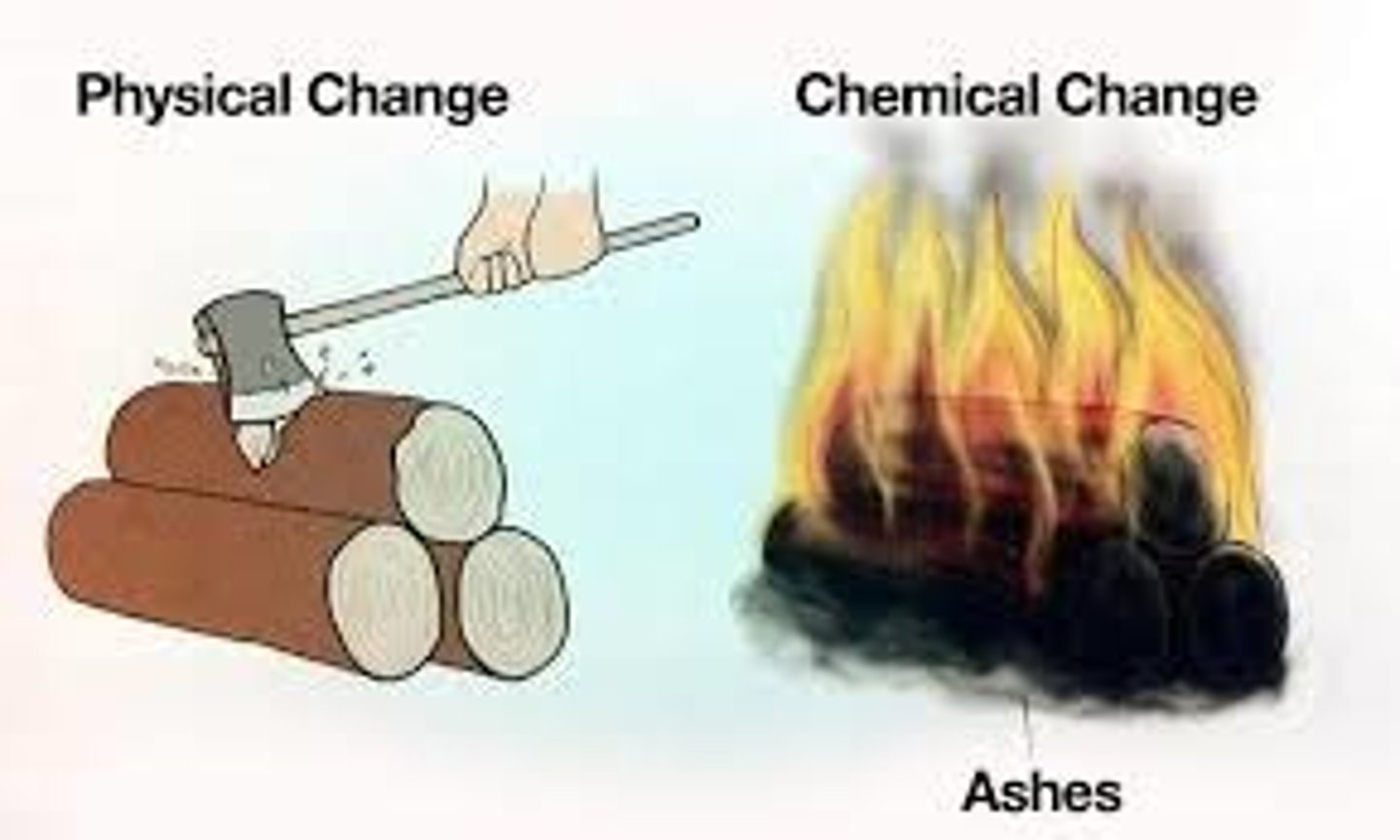
chemical property
A characteristic of a substance that can only be observed during a chemical reaction.

reactants
The substance that enter into a chemical reaction
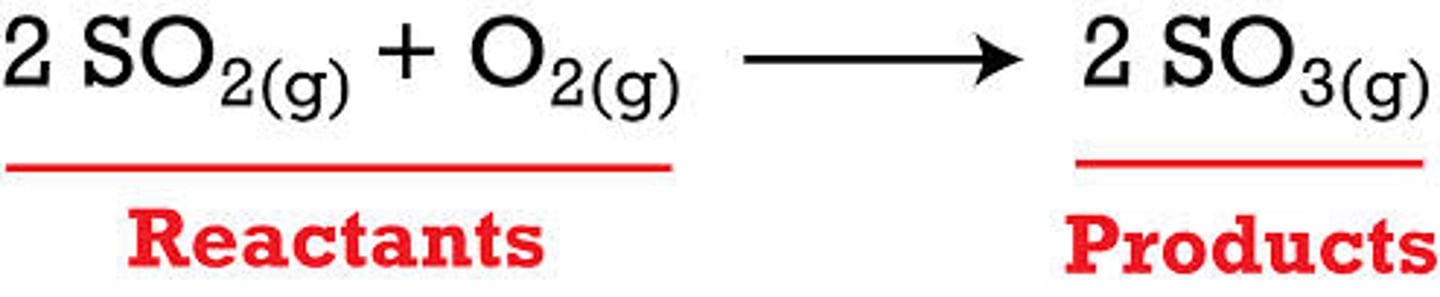
products
A substance formed as a result of a chemical reaction.
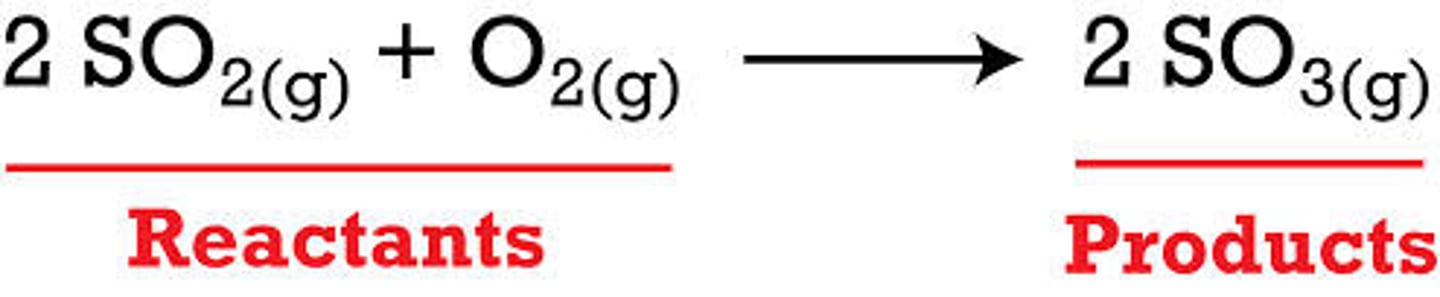
exothermic reaction
A reaction that releases energy in the form of heat (new substance feels warmer)
endothermic reaction
A reaction in which energy is absorbed (new substance feels colder)
closed system
A system in which no matter is allowed to enter or leave
precipitate
A solid that forms from a solution during a chemical reaction.

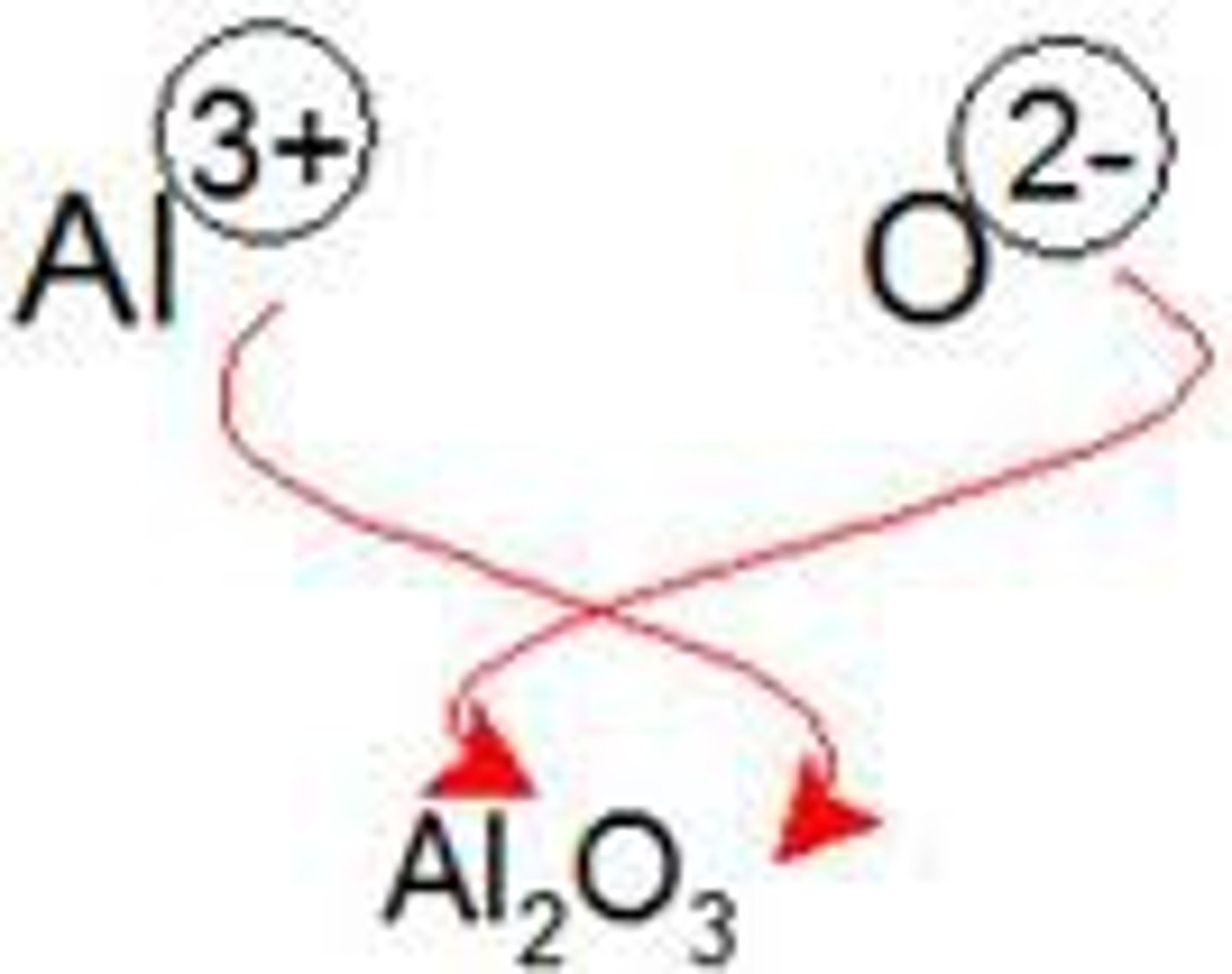
chemical bond
the force that holds two atoms together
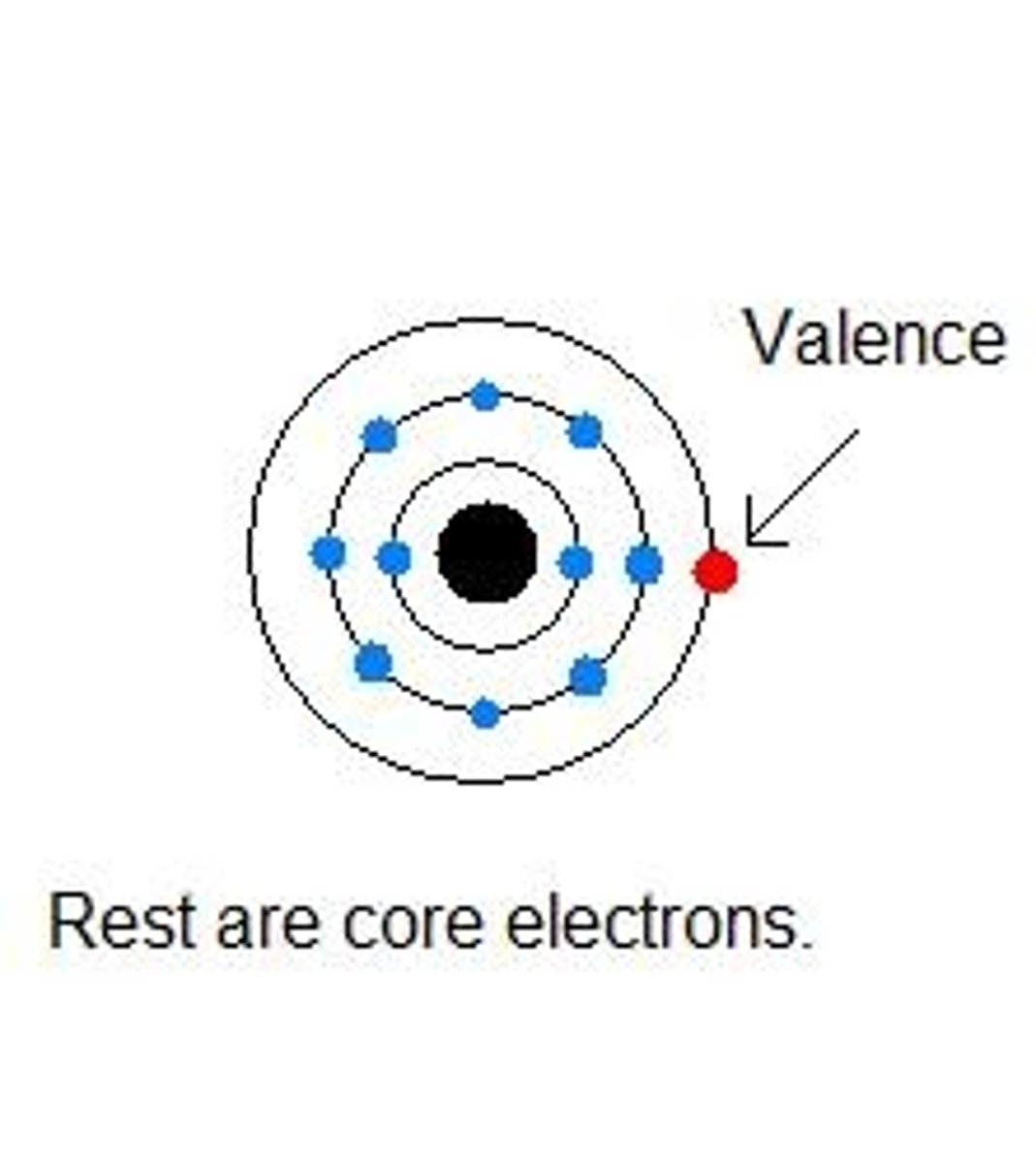
valence electrons
Electrons on the outermost energy level of an atom
octet rule
States that atoms lose, gain or share electrons in order to acquire a full set of eight valence electrons

Law of Conservation of Mass
the law that states that mass cannot be created or destroyed in ordinary chemical and physical changes
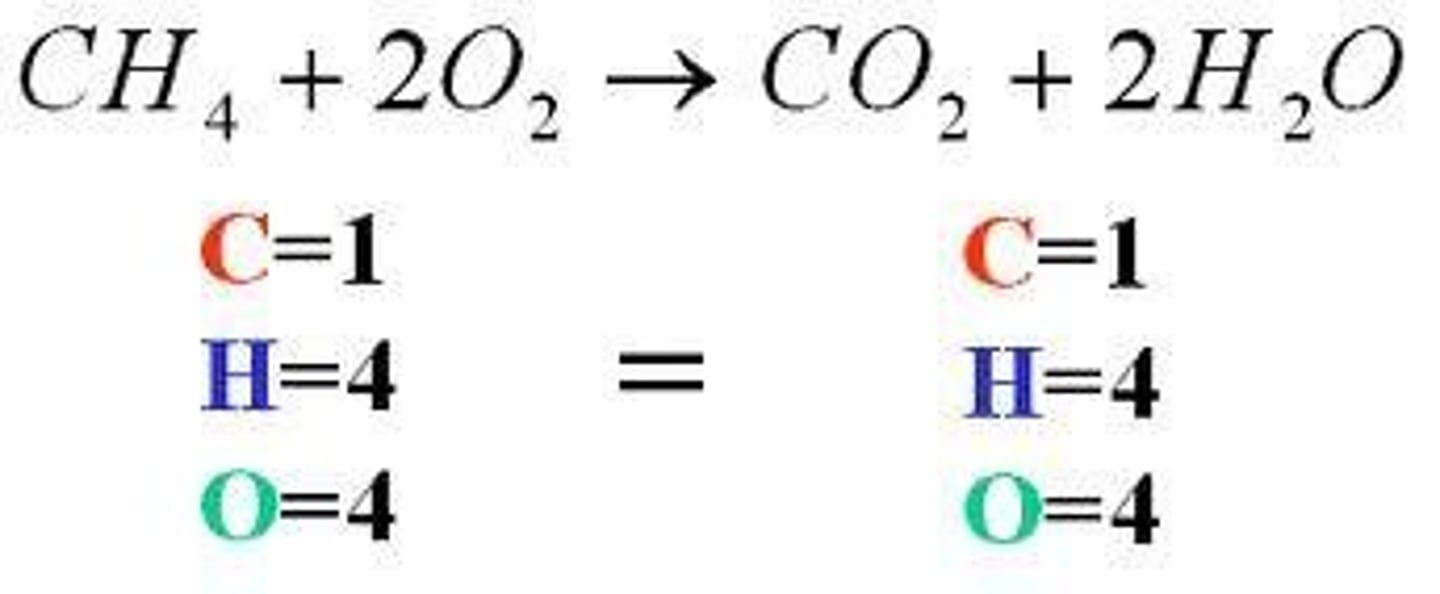
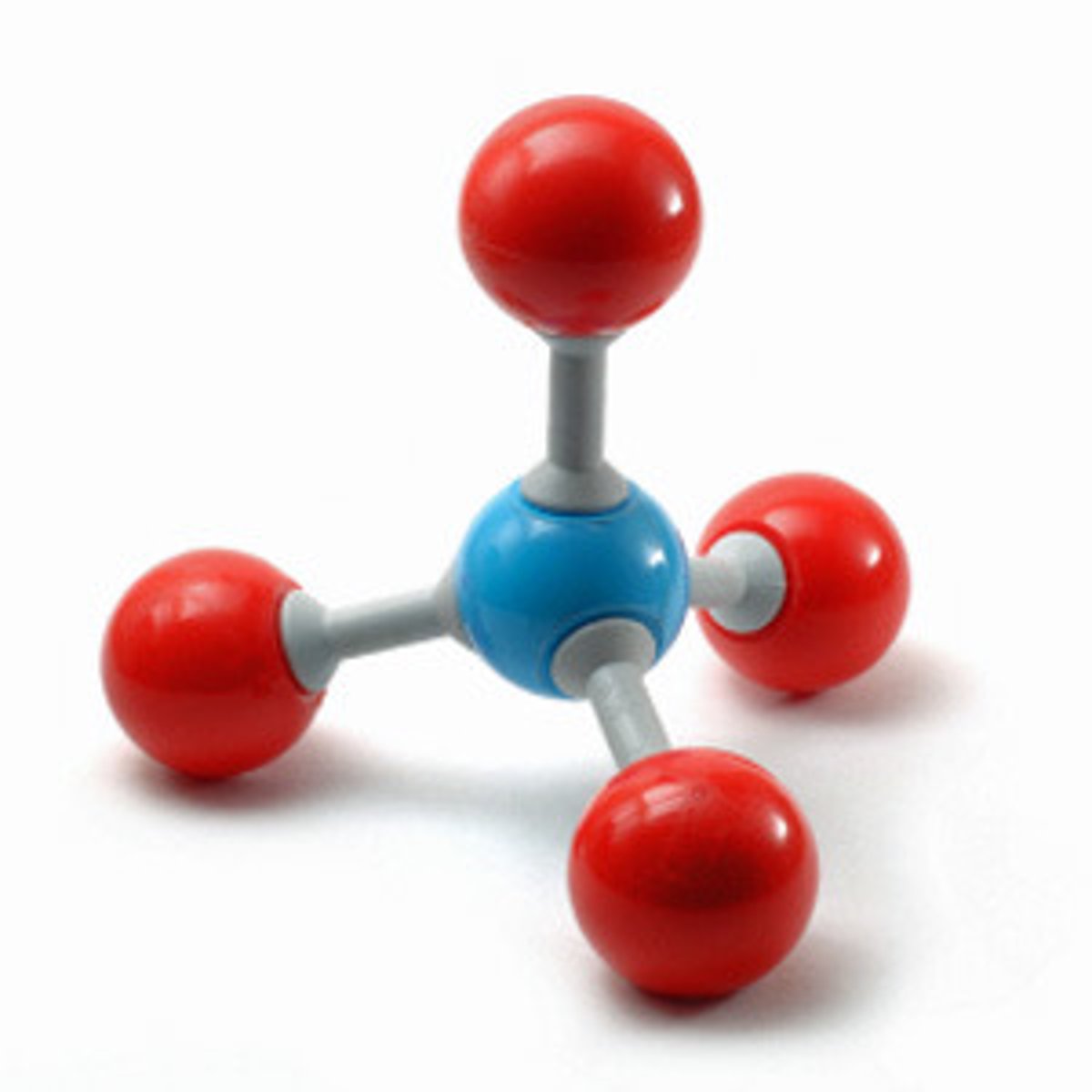
oxidation
A chemical change in which a substance combines with oxygen, as when iron oxidizes, forming rust
flammable
A chemical property of matter

chemical reaction
the process by which one or more substances change to produce one or more different substances