Matter, Trends, & Bonding - Isotopes and Periodic Trends
0.0(0)
Card Sorting
1/22
Last updated 2:06 PM on 12/12/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
1
New cards
Radioisotopes
Isotopes that have unstable nuclei and undergo radioactive decay.
2
New cards
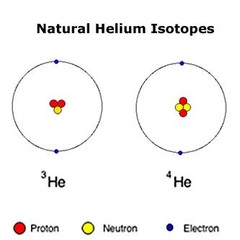
Isotopes
Atoms that contain the same number of protons but different number of neutrons, creating various mass numbers for one type of element.
3
New cards
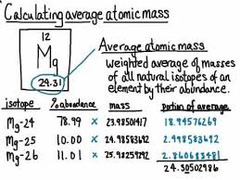
AAM formula
AAM = (mass)(%)+(mass)(%)
(convert all percentages into decimals)
EX:
chlorine-35,76% and chlorine-37,24%
AAM = (35)(0.76)+(37)(0.24)
(convert all percentages into decimals)
EX:
chlorine-35,76% and chlorine-37,24%
AAM = (35)(0.76)+(37)(0.24)
4
New cards
Nuclear fission
A nuclear reaction in which a massive nucleus splits into smaller nuclei with the simultaneous release of energy
5
New cards
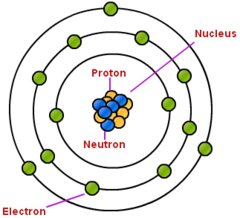
Subatomic particles
protons, neutrons, electrons
6
New cards
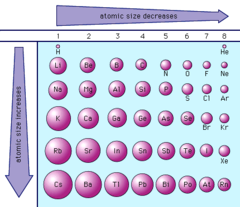
Atomic radius (AR)
the distance from the centre of an atom to the boundary within which the electrons spend 90% of their time.
7
New cards
Ionization energy (IE)
The amount of energy required to remove an electron from the outermost orbit of an atom.
8
New cards
Does atomic radius increase or decrease going across a period?
Decreases. As positive charge increases as you go across a period, the attraction grows stronger between the protons and orbiting electrons. This pulls them closer to the nucleus, thus decreasing the radius.
9
New cards
Does atomic radius increase or decrease going down a group?
Increases. More oribitals are added as you go down a group, making the radius larger.
10
New cards
Does ionization energy increase or decrease going down a group?
Decreases. With more orbitals, less energy is needed to remove the electrons that are far from the protons that attract them.
11
New cards
Does ionization energy increase or decrease going across a period?
Increases. Non-metals (located on the right of the periodic table) want to GAIN electrons to achieve a stable octect, not lose them, meaning it would take a lot of energy to pull away the electrons.
12
New cards
Electron affinity (EA)
The energy absorbed or released when an electron is added to a neutral atom.
13
New cards
Electronegativity (EN)
An indicator of the ability of an atom to attract shared electrons more closely to itself.
14
New cards
Does it require less or more energy to remove the electron in an atom's 2nd ionization energy?
More energy.
15
New cards
What happens if energy is absorbed after an electron is added to a neutral atom?
The resulting ion would be unstable.
16
New cards
Does electron affinity increase or decrease in negativity going across a period?
Increases until noble gases are reached; elements on the right side of the PT, especially the halogens, want to obtain more electrons (aka, become more negative).
17
New cards
Does electron affinity increase or decrease in negativity going down a group?
As the orbitals increase, the valence electrons are further away from the nucleus, meaning there is less of an attraction between them and less energy is released.
18
New cards
Does electronegativity increase or decrease going across a period?
Increases. Elements on the right want full valence shells, so they are better at attracting electrons.
19
New cards
Does electronegativity increase or decrease going down a group?
Decreases; the atomic radius is larger towards the bottom of a group, so electrons are less attracted to the nucleus.
20
New cards
What is the Pauling scale?
a scale for measuring electronegativity
21
New cards
Periodic law
When elements are organized by atomic number, many of the physical/chemical properties reoccur.
22
New cards
Picometer (pm)
10 -12 m
23
New cards
Effective nuclear charge
The actual amount of positive (nuclear) charge experienced by an electron in a multi-electron atom.