FMT Term 2
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
Define the terms; element, atom, matter, proton, neutron, electron, nucleus, electron shell, electron arrangement.
Element: a substance made up of atoms with the same number of protons, and cannot be broken down into a simpler substance.
Atom: the smallest particle of an element that can exist, and is neutrally charged.
Matter: any physical substance that has mass and occupies space.
Proton: a stable subatomic particle found in the nucleus and has a positive charge while weighing ~1 AMU
Neutron: a stable subatomic particle found in the nucleus and has a neutral charge while weighing ~ 1 AMU
Electron: a stable subatomic particle found in the outer shells and has a negative charge, while weighing a negligible mass.
Nucleus: the small dense region at the centre of an atom/ion, consisting of protons and neutrons and making up almost all of the atom/ion's mass.
Electron shell: an orbit that electrons follow around the atom/ion's nucleus (AKA energy level)
Electron arrangement: how the electrons are ordered in each of the shells (e.g. silicon = 2, 8, 4)
Sketch the structure of an atom (Bohr-Rutherford), showing the nucleus and electron shells.
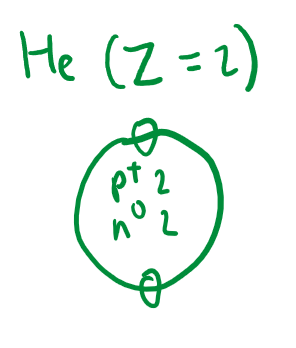
Determine and state: the mass, charge and location of subatomic particles (protons, neutrons and electrons).
| Proton | Electron | Neutron |
Mass | ~1 AMU | ~0 | ~1 AMU |
Charge | Positive | Negative | Neutral |
Location | Nucleus | Outer Shell | Nucleus |
Investigate the development of the atomic model
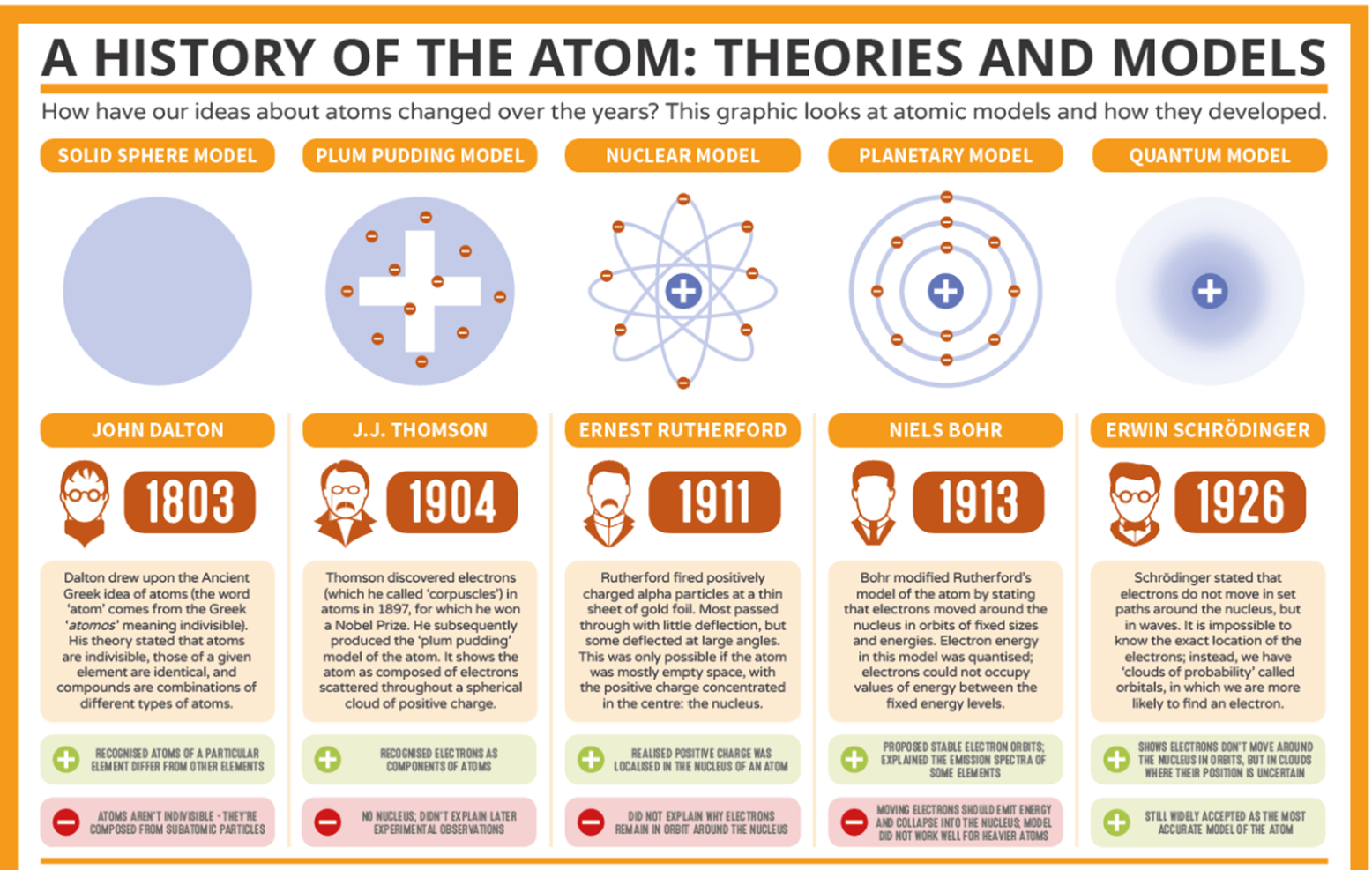
Describe the 'Periodic Law'
The periodic repetition of physical and chemical properties of elements when arranged with respect to atomic number.
Define the following terms; periodic law, group, period, atomic number, ion, atomic radius, ionic radius, valence electrons
Periodic law: the periodic repetition of physical and chemical properties of elements when arranged with respect to atomic number
Group: columns on the periodic table arranging elements by their number of valence electrons; elements of the same group have similar physical and chemical properties
Period: rows on the periodic table arranging elements by their number of electron shells / energy levels
Atomic number: the number of protons within an atom
Ion: an atom who has either lost or gained electrons, thus yielding a net electric charge
Atomic radius: the distance between an atom's nucleus and valence shell
Ionic radius: the distance between an ion's nucleus and valence shell
Valence electrons: electrons located on the valence (outermost) shell
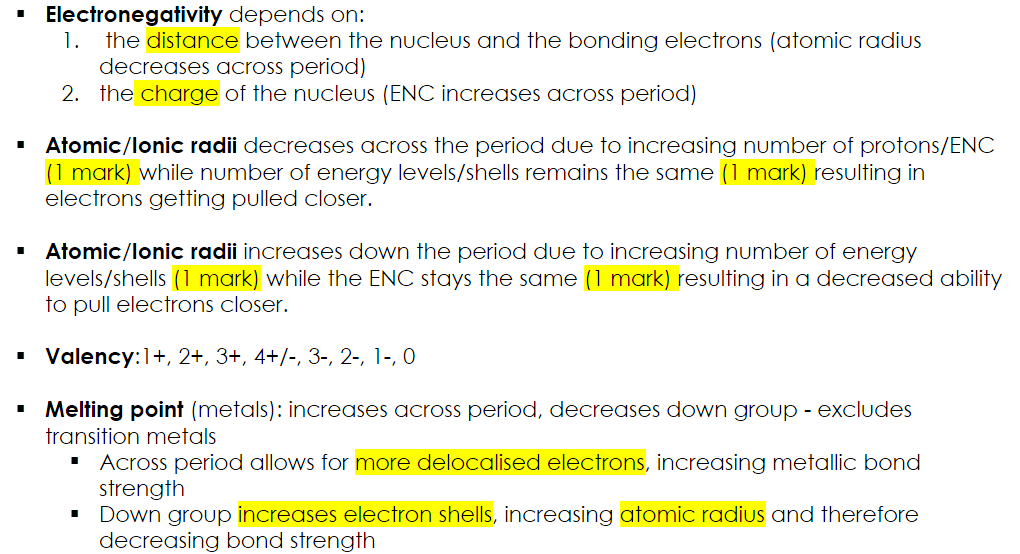
Explain the periodic trends in; atomic/ionic radii, valency (groups), and melting point (metals only) across periods and within groups in the Periodic Table.
Electronegativity depends on:
the distance between the nucleus and the bonding electrons (atomic radius decreases across period)
the charge of the nucleus (ENC increases across period)
Atomic/Ionic radii decreases across the period due to increasing number of protons/ENC (1 mark) while number of energy levels/shells remains the same (1 mark) resulting in electrons getting pulled closer.
Atomic/Ionic radii increases down the period due to increasing number of energy levels/shells (1 mark) while the ENC stays the same (1 mark) resulting in a decreased ability to pull electrons closer.
Valency:1+, 2+, 3+, 4+/-, 3-, 2-, 1-, 0
Melting point (metals): increases across period, decreases down group - excludes transition metals
Across period allows for more delocalised electrons, increasing metallic bond strength
Down group increases electron shells, increasing atomic radius and therefore decreasing bond strength
Predict; the relative size of an atom or ion, number of valence electrons of an atom, charge of ion
A group 1, 2, 13 element's atom is larger than its ion. This is because the ion loses a shell, decreasing the number of the shielding electrons while the number of protons stays the same. This means the ENC increases, decreasing the ionic radius
Contrarily a group 6, 7 element's atom is smaller than its ion. This is because the number of electrons increases, while the number of protons remains the same, increasing the ionic radius as the nucleus has a weaker ability to pull in valence electrons.
Explain how the relative electronegativities of elements involved in a chemical bond will result in a specific type of bond
Electronegativity differences:
0 - 0.4 = non-polar covalent bond
0.4 - 1.7 = polar covalent bond
1.7+ = ionic bond
Higher differences in electronegativity -> more unequal electron sharing -> permanent dipole moments characteristic of polar covalent bonds.
More electronegative atom attracts electrons more strongly -> creating partial positive and negative charges.
In contrast, ionic bonds involve the complete transfer of electrons from one atom to another ->a stronger electrostatic attraction between the oppositely charged ions.
Define; ionic bonding, giant ionic lattice
Ionic bonding: a linkage formed from the electrostatic attraction between oppositely charged ions, consisting of a metal and a non-metal. Element with lower electronegativity transfers valence electron(s) to more electronegative element.
Giant ionic lattice: formed in all ionic compounds, refers to their uniformly arranged and symmetrical structure of alternating anions and cations (in solid state), yielding a net neutral charge with very strong bonds.
High melting and boiling points
High strength & hardness, low ductility
Brittle (any slight displacement results in repulsion between oppositely charged ions)
Polar, meaning they are highly soluble in water
Not conductive in solid, conductive as a liquid
Explain physical properties such as; melting point, solubility, conductivity and brittleness with respect to ionic bonding.
Melting point: HIGH because the electrostatic attraction between oppositely charged ions is strong, requiring a high amount of energy to overcome.
Solubility: HIGH because ionic compounds are polar; sufficient attractive forces from the partial charges of water molecules break the bond. Cation attracted to negatively charged oxygen, while anion attracted to positively charged hydrogen.
Conductivity: LOW when solid; very strong bonds make it hard for the ions to move and transfer energy… HIGH when liquid/aqueous; bonds are broken, cations and anions are free to move, thereby allowing electricity and kinetic energy to flow more efficiently.
Brittleness: HIGH because any slight displacement in the structure causes ions of the same of the same charge to be adjacent, which then repulse from each other.
Define metallic bonding
Consisting of one or more types of metals, the bonding between two metals due to the electrostatic attraction between metal cations and delocalised electrons.
Construct and annotate a diagram depicting the relationship between metal nuclei and delocalised electrons.
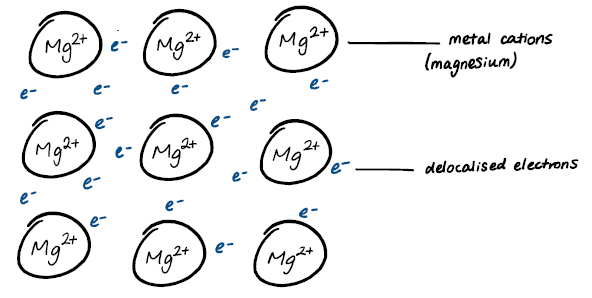
Describe crystal structure in solid metals.
Metals have a tendency to arrange in the closest possible arrangement
3D lattice structure of metal cations with delocalised electrons
Different structures (e.g. BCC, FCC, BCT, HCP)
Each have different coordination numbers (number of cations connected to any single cation)
Each have different slip planes due to atomic packing/density
Describe annealing in metals
Involves rapidly heating the metal/alloying to above recrystallisation temperature, then cooling very slowly in a furnace creating a very slow rate of solidification, resulting in larger grains, relieving dislocations, and increasing ductility while decreasing strength
Explain typical metal properties such as; strength, malleability, ductility, high conductivity, high melting point, in relation to metallic bonding.
Malleability: metals have high malleability as the cations in the lattice can easily slide over each other without the bonds being broken, as the electrons in which the cations are bonded to are delocalised and can move, meaning that the shape of the metal can be manipulated easily without fracturing.
Ductility: high; follows the same principle as malleability, but refers to the metal being easily stretchable.
Strength: metals have high strength (ability to resist applied force before failure or plastic deformation) due to the strong electrostatic forces between the oppositely charged particles of metal cations and delocalised electrons.
Conductivity:
Thermal: metals have high thermal conductivity as the delocalised electrons can transfer kinetic energy throughout the material efficiently due to them being free moving, which allows them to vibrate vigorously throughout the whole structure.
Electrical: metals have high electrical conductivity as the delocalised electrons are free to move, meaning they can flow when an electrical current is passed through.
Melting point: similar to strength, metals have high melting points due to their strong bonds, which are formed due to the strong electrostatic attraction between delocalised electrons and metal cations, meaning a high temperature is required to overcome these bonds.
Define; covalent bonding, electronegativity.
Covalent bonding: a linkage between two atoms formed by the sharing of electron pair(s), resulting in an electrostatic attraction between the positively charged nuclei and the shared electrons.
Electronegativity: a measure of the tendency for an atom to attract a bonding pair of electrons in a chemical bond, which depends on:
the atomic radius (distance between valence shell and nucleus)
the effective nuclear charge.
Explain the behaviour of electrons in non-polar covalent, polar covalent, and ionic bonding.
Non-polar covalent: non-metals, electrons are shared equally in the covalent bond, resulting in no dipole moment.
Rely on dispersion forces to form intermolecular bonds formed by instantaneous poles that arise by chance when electrons wiz around the atoms existing only for an instant
Polar covalent: non-metals, electrons are shared unequally in the covalent bond, resulting in a dipole moment.
Dipole moment results in electrons being more attracted to the more electronegative atom, creating a partial positive and negative charge in the bond
Ionic: a metal and a non-metal, entire electrons are transferred from the less electronegative to the more electronegative atom, resulting in a permanent electrostatic attraction between oppositely charged ions
Explain that there is a spectrum of bonding from ionic to covalent in relation to electronegativity.
As the difference in electronegativity increases, the ionic character increases.
Annotate bond dipoles and bond polarities on diagrams of molecules.
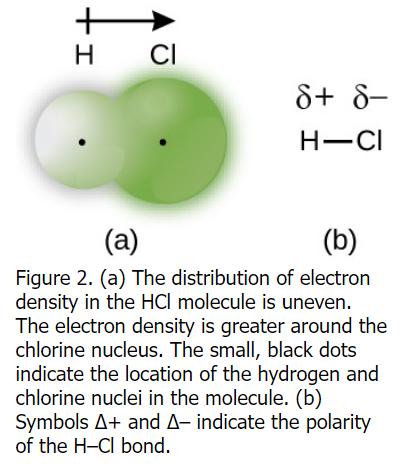
Explain how polar covalent bonds can cause intermolecular forces.
The partial positive and partial negative charges in the polar covalent bond result in dipole-dipole interactions forming, a type of intermolecular force that creates a weak electrostatic attraction between the partially positively charged end and partially negatively charged end of two polar covalent bonds.
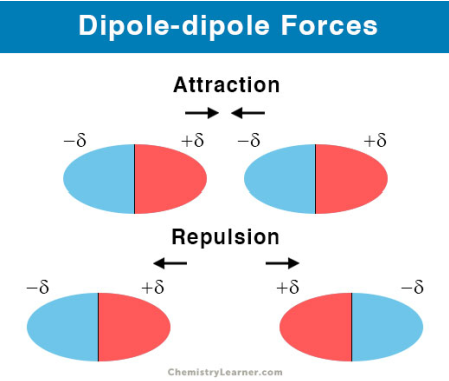
Deduce the relative boiling points, or solubility in water, of similar compounds with respect to non-polar or polar covalent bonding.
Non-polar is not soluble, whereas polar is soluble in water due to the interaction between their poles, overcoming intermolecular forces
Non-polar have lower boiling points due to their weaker intermolecular forces (dispersion forces), whereas polar covalent have stronger intermolecular forces (dispersion + dipole-dipole interaction)
Define the following terms: monomer, polymer, polymerisation
Monomer: any molecule that can chemically react with other molecules to form very large molecules
Polymer: a very large molecule made up of many chemically bonded monomers
Polymerisation: the process of monomers chemically reacting to form polymers
Describe the role and factors that influence of secondary intermolecular forces in the context of polymers.
Distance / density, altered by branching
Length / number of monomers in polymer chain
Number of intermolecular cross-links
Polarity / specific intermolecular bond present
Describe properties given to polymers through amorphous and crystalline regions.
Amorphous regions are packed and arranged less efficiently, decreasing density and therefore strength while increasing flexibility
Crystalline regions are packed and arranged in uniform order and very efficiently, increasing density and therefore strength while decreasing flexibility
Semicrystalline substances have some amorphous and some crystalline arrangements/regions
Branching can affect this, and this amorphous vs crystalline effect can be observed in LDPE vs HDPE
Describe what is meant by chemical resistance for polymers
Refers to the ability of a polymer to withstand against damage, degradation, or chemical change when exposed to various chemicals due to the non-polar nature of their bonds.
Explain why polymers can be effective insulators of electricity and heat.
Polymers are good electrical insulators because the electrons are tightly held together by the strong covalent bonding, meaning that there are no free electrons to carry an electric current
Polymers are good thermal insulators because the strong covalent bonds between monomers restricts the ability for vibration of the atoms, therefore stopping the spread of kinetic energy (temperature)
Explain how; polymer chain length, branching, and cross-linking can affect physical properties such as flexibility, density and melting point.
Polymer chain length:
Longer polymers become more entangled and packed together
Increases density slightly (dispersion forces slightly make chains closer together)
Increases number of intermolecular forces (dispersion), thus increasing melting point slightly
Entanglement further restricts the movement of the polymer chains (via dispersion forces), thus decreasing flexibility (but increasing strength)
Branching:
more branching in polymers decreases crystallinity while increasing the distance between polymer chains
Decreases density; polymer chains are spread further apart
Greater distance decreases the strength of intermolecular forces, thus decreasing melting point
Greater distance increases free volume throughout the structure, creating more space for the polymer to move and deform and therefore increasing flexibility (while decreasing strength)
Cross-linking:
more cross-linking in polymers increases the interconnectedness of the network (whether physical or chemical)
Increases density; more interconnectedness (amplified with chemical crosslinking)
More intermolecular bonds (physical) and/or chemical bonds (chemical), increasing melting point
More rigid structure, restricting movement of polymer chain and therefore decreasing flexibility (greater effect in chemical) (but increasing strength)
Discuss why polymers are so useful with respect to specific properties.
Their physical properties are easily manipulable (e.g. HDPE and LDPE), allowing for the properties (e.g. flexibility, strength,
melting point) to be tailored to specific use cases
Electrical and thermal insulation, allowing them to be used as protection around wires (PVC) or to conserve heat (polystyrene)
Chemical resistance, as they can resist degradation and damage, making them durable even in harsh conditions
They are very cheap and easy to manufacture
Identify and describe addition and condensation polymers.
Addition polymers are formed due to the breakage of a C=C double bond, meaning it is a radical as it has a free electron that it wants to use to bond with more monomers, creating long chains of C-C bonds
Condensation polymers are formed due to the bonding of functional groups on either side of a carbon chain (R), which also results the removal of a small molecule, typically being water
Sketch and annotate addition and condensation polymers. Identify the key feature of monomers for the formation of addition polymers and condensation polymers.
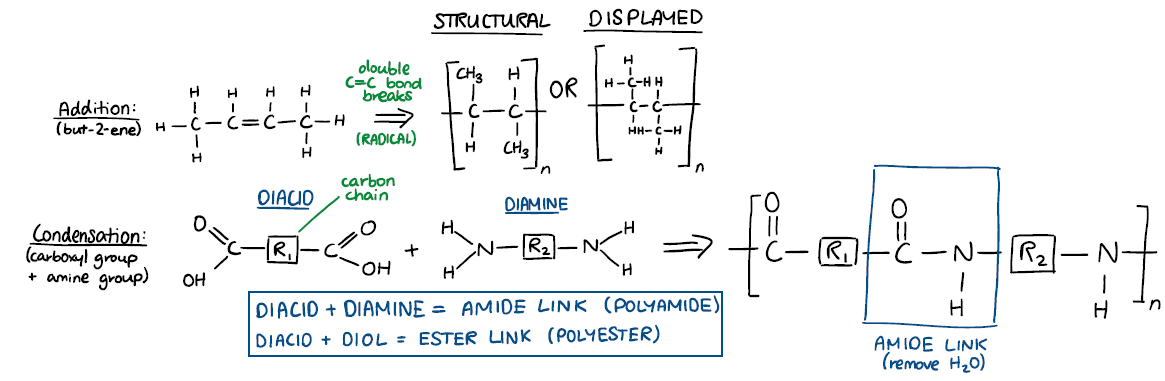
Describe and use chemical reactions to demonstrate addition polymerisation and condensation reactions for polyalkenes, polyesters, and polyamides (specific reaction conditions not required)
Addition: alkene, C=C bond breaks to make a radical, radical joins other radicals creating large polymer chain
Condensation: diacid + diamine/diol, HO is removed from carboxyl group and H is removed from diamine/diol, amide/ester linkage is formed creating the polymer, R indicates a carbon chain of variable length in between the two functional groups
Classify plastics as conventional or bio-based.
Conventional plastics are made through using fossil fuels (i.e. crude oil)
Bio-based plastics are made through renewable biomass resources (e.g. corn starch)
Describe biodegradability.
Biodegradability is the ability for a substance to be broken down by bacteria and other organisms, avoiding pollution; ability to decay naturally without harm
Discuss societal and environmental implications of the production, use, and post-use, of plastics

Identify the following classes and functional groups: alkanes, alkenes, alcohols, carboxylic acids, amines.
Class | Functional Group |
Alkane | none |
Alkene | Double carbon bond |
Alcohol | Hydroxyl (-OH) |
Amine | Amino (-NH2) |
Carboxylic acid | Carboxyl (-COOH) |
Name organic compounds of the classes above, 1-6 C, using IUPAC rules of organic nomenclature.
Prefixes:
Meth-, eth-, prop-, but-, pent-, hex-
Number in middle or at start to indicate position of functional group
An to indicate no double carbon bond
Suffix:
-ane, -ene, -ol, -amine, -noic acid.
Evaluate, discuss, compare and contrast physical properties of substances with covalent, ionic and metallic bonding.
| Strength | Ductility | Melting Point | Solubility | Thermal Conductivity | Electrical Conductivity |
Covalent (molecular) | Low | High | Low | Only polar molecules | Poor | Poor |
Covalent (network) | Very high | Low | Very high | Insoluble | Poor | Generally low |
Ionic | Very high | Low | Very high | Soluble | Low/high | Low/high |
Metallic | High | High | High | Insoluble | High | High |
Define a molecule
A group of two or more discretely covalently bonded atoms, forming the smallest unit of a substance.
Define network solids
A type of solid in which its atoms are linked together by a continuous network of covalent bonds
Deduce the type of bonding in a substance based on properties (ionic, metallic, covalent molecular, covalent network solid)
