Chemistry GCSE Mocs
1/50
Earn XP
Description and Tags
Particle model, Chemical or physical change, Atomic structure, Discovery of atomic structure, Isotopes and ions, Metals and Non-metals, Periodic table
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
Solid Characteristics
Cant move, Touching, Vibrate, No space between, Fixed volume, Fixed shape ,Large force between
Liquid Characteristics
Can move, Touching, No space between, fixed volume, no fixed shape, Weak force between
Gas Characteristics
Moving quickly, Not touching, far apart, no fixed volume, no fixed shape, weak force between
The particle model doesn’t take into account of…
Forces between, 3D aspect, The size, The space between
Solid to Gas
Sublimation
Gas to solid
Deposition
Solid to liquid
Melting
Liquid to gas
Evaporation
Gas to liquid
Condensation
Liquid to solid
Freezing
Chemical change
A change that produces a new substance
Physical change
A change that is reversible
An atom is made up of …
Electrons, Neutrons and protons
What did the Ancient Greeks propose out about the atomic structure
Everything was made up of an atom which is the building blocks of everything
What did Dalton propose
Each element has its own type of atom and atoms are just spheres.
What did J.J Thompan propose
The plum pudding model and negatively charged electrons.
Who did the Gold Foil Experiment
Rutherford, Gieger, Marsden
What is a Isotope
Different forms of the same element that have the same number of protons but a different number of neutrons
What is a Ion
An atom that has gained or lost electrons to become negatively or positively charges
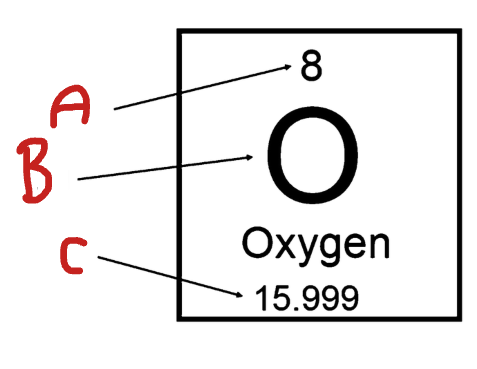
What is A
Atomic Number
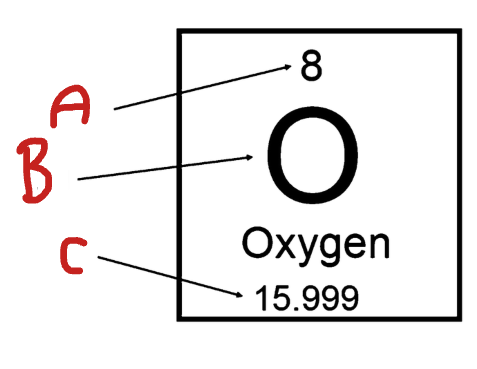
What is B
Element Symbol
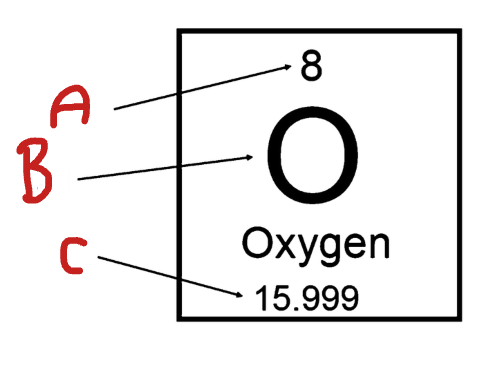
What is C
Atomic Mass
What is the atomic number
The number of protons in an atom
What is the atomic mass
The number of protons and neutrons in an atom
What is a Cation
A atom with a positive charge
Typical properties of Non-metals
Any state at room temp, not shiny, don’t conduct electricity, Don’t Conduct heat, brittle, not ductile
Typical properties of metals
Solid at room temp, shiny if polished, conduct electricity, conduct heat, malleable, ductile
Metal oxides are Acidic/Alkaline - select one
Alkaline
Non-metal oxides are Acidic/Alkaline - select one
Acidic
What is Ionic bonding (Exam Question)
Strong electrostatic forces cause electrons to transfer from a metal to a non-metal
What are covalent bonds
Where atoms share their outer shell electrons to achieve a full shell
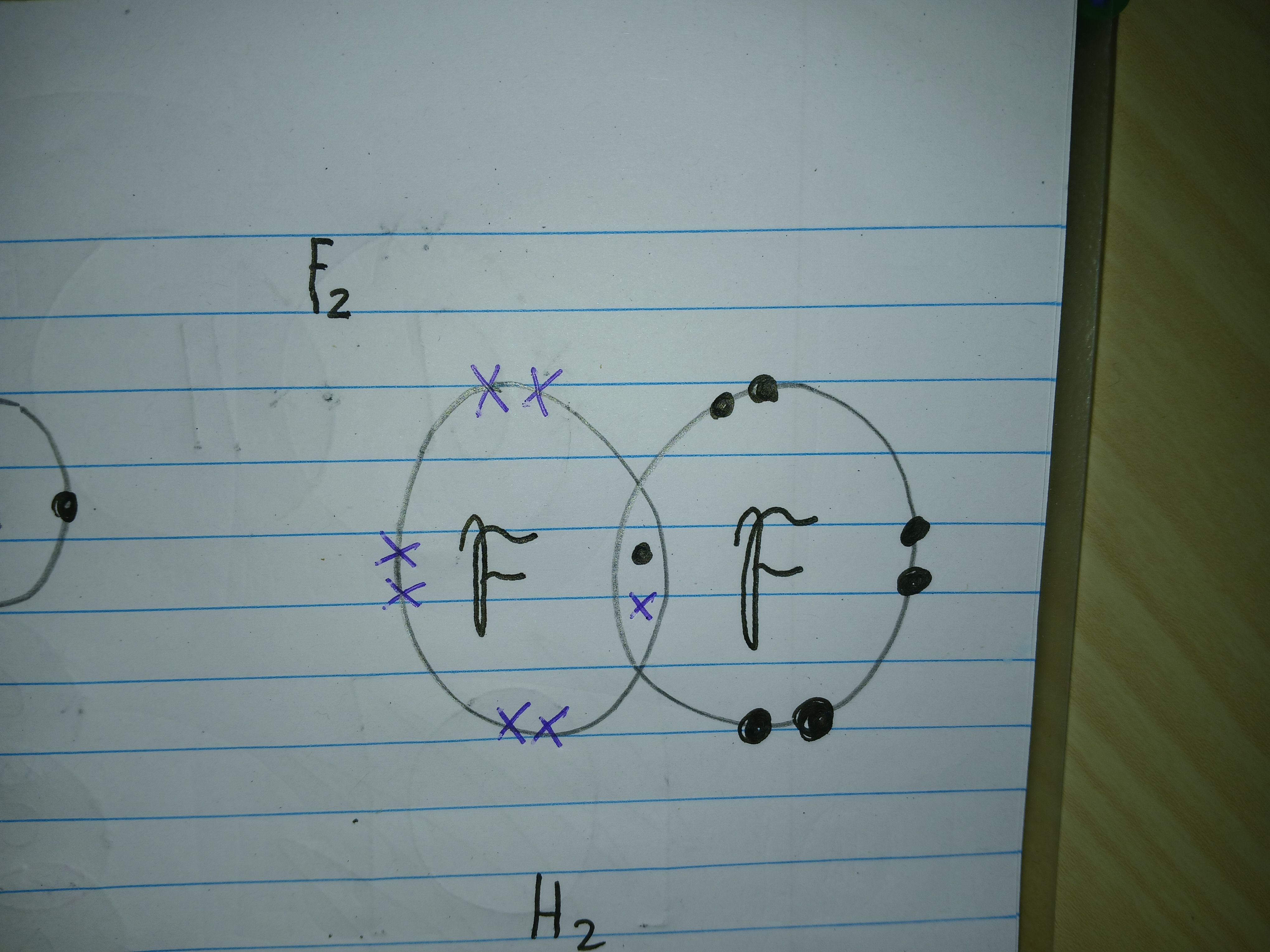
What bond is this
Covalent bond
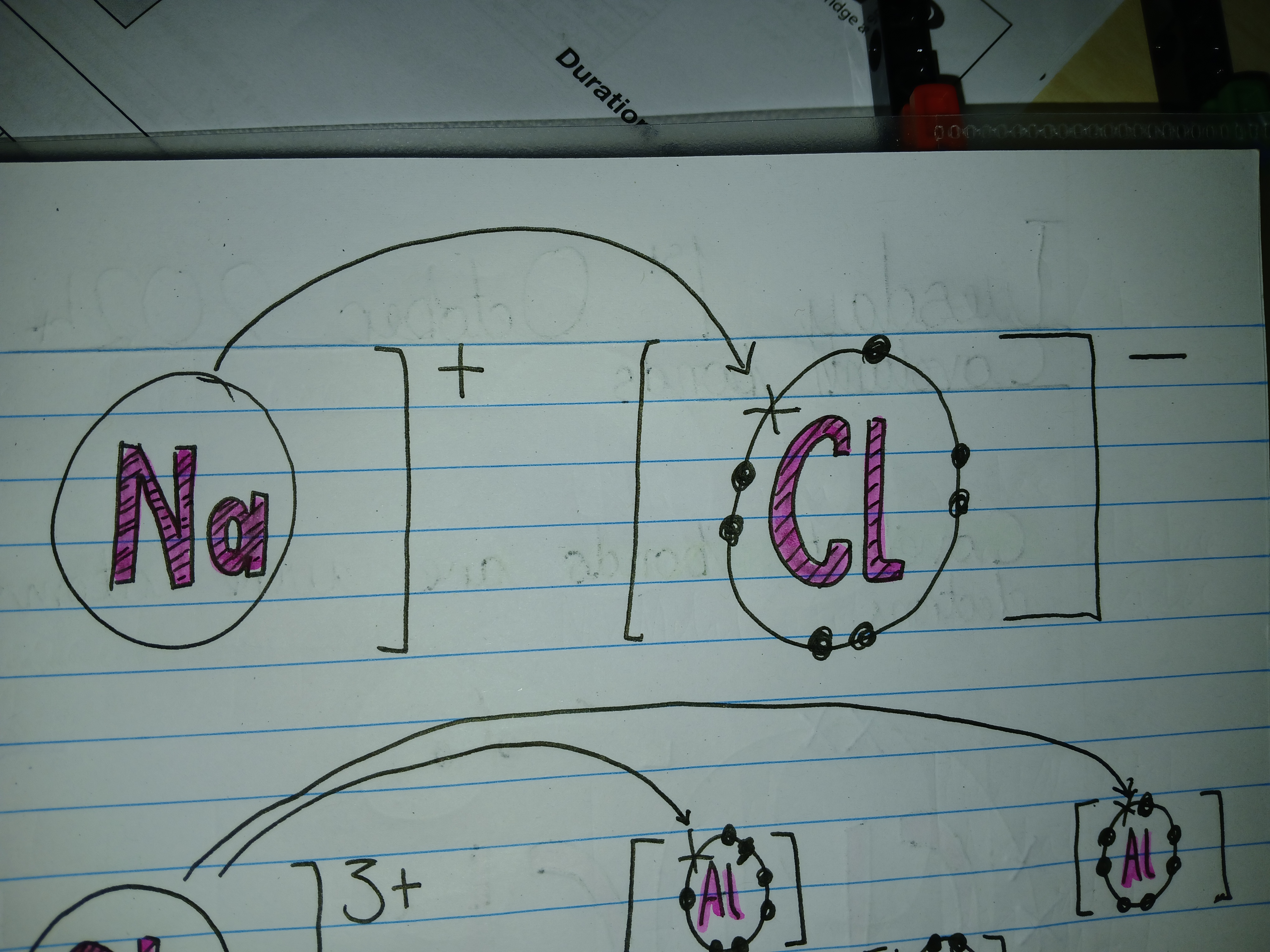
What bond is this
Ionic bond
What element is H
Hydrogen
What element is Li
Lithium
What element is Be
Beryllium
What element is Na
Sodium
What element is Mg
Magnesium
What element is K
Potassium
What element is Ca
Calcium
What element is Sc
Scandium
What element is Rb
Rubidium
What element is Sr
Strontium
What element is Y
Yttrium
What element is Cs
Caesium
What element is Ba
Barium
What element is La
Lanthanum
What element is Fr
Fransium
What element is Ra
Radium
What element is Ac
Actinium
What are allotropes
different forms of the same element and same state but different atomic arrangements