Biology A-Level: Required Practicals 1-6
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
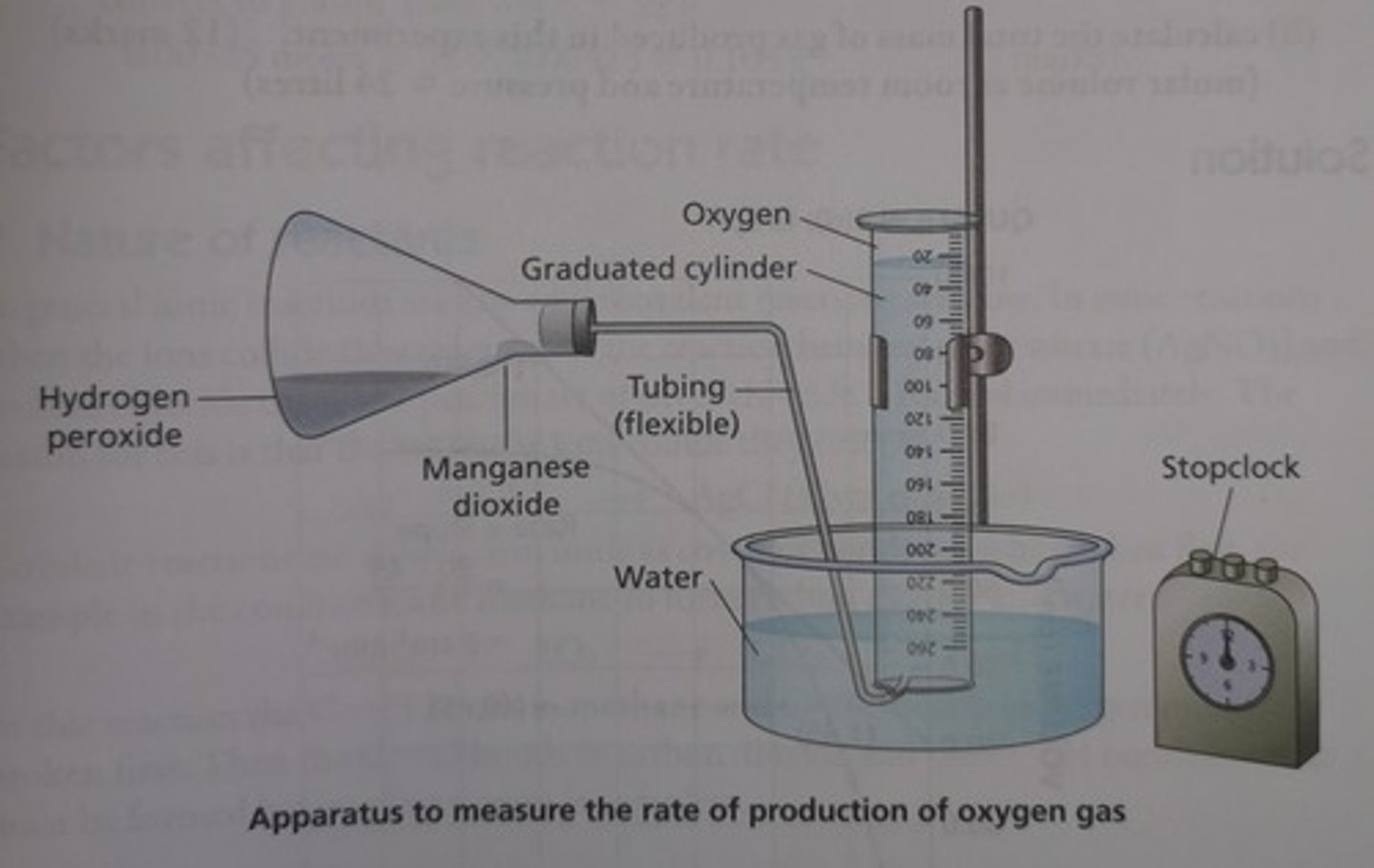
Outline a method for measuring the rate of an enzyme-controlled reaction (RP1)
1) Set up boiling tubes with same volume and conc. hydrogen peroxide
- to keep pH constant, add equal volumes of buffer solution to each tube
2) Set up apparatus in similar fashion to image (boiling tube replaces conical flask)
3) Place each boiling tube in a water bath set to a different temp along with tube containing catalase
- allow catalase to equilibrate for 5 mins
4) Use pipette to add set volume of catalase to boiling tube at lowest temp.
- quickly attach bung and delivery tube
5) Record volume of O2 produced in first minute (time with a stopwatch)
6) Repeat experiment 3 times at each temp.
- calculate mean volume of O2 produced for each temp
7) Calculate mean rate of reaction at each temp by dividing volume of O2 by time taken (60 secs)
Outline a method for preparing a root tip cell squash (RP2)
1) Add 5 mm depth of 1M HCl to a boiling tube
- enough to cover root tip
- place tube in 60°C water bath and leave to equilibrate
2) Use scalpel to cut 1cm from the tip of a growing root
3) Transfer the root tip to the boiling tube containing acid and incubate for 5 mins
4) Use tweezers to remove root tip from tube and rinse with cold water
- leave to dry on paper towel
5) Place root tip on a microscope slide and cut 2mm from the tip
6) Use a mounted needle to spread the cells of the tip thinly
7) Add a few drops of stain solution and leave for a few mins
- stain will make chromosomes more visible under the microscope
8) Place cover slip over cells and place folded filter paper on top
- push down firmly to squash the tissue
- squashing makes the tissue thin to allow light to pass through
- don't smear cover slip sideways, because chromosomes may be damaged
Outline how to use an optical microscope (RP2)
1) Clip slide prepared onto stage
2) Select lowest-powered objective lens
3) Use coarse adjustment knob to raise the stage to just below the objective lens
4) Look down the eyepiece and use the coarse adjustment knob to move the stage and focus the image
5) To increase magnification, repeat the process with a higher-powered objective lens
Outline how to calculate the actual size of cells under a microscope (RP2)
1) Eyepiece graticule is fitted onto the eyepiece
- calibrate the eyepiece graticule using a stage micrometer
2) Measure the cells using the eyepiece graticule
- calculate the actual size of the cells using the magnification and calibration with the stage micrometer
3) Repeat measurements multiple times to ensure value is accurate
Outline a method for producing a dilution series of a sucrose solution (RP3)
1) Line up 5 test tubes in a rack
2) Add 10cm3 of 2M sucrose solution to first test tube and 5cm3 distilled water to other test tubes
3) Draw 5cm3 of solution from first test tube and add it to the distilled water in the second test tube
- mix solution thoroughly
4) Repeat with remaining test tubes
- will form 5 solutions with following concentrations:
2M, 1M, 0.5M, 0.25M, 0.125M
Outline a method for investigating the effect of temperature on membrane permeability (RP4)
1) Use a scalpel to cut 5 equal sized pieces of beetroot
- rinse the pieces to remove any pigment released during cutting
2) Put each piece in a different test tube, each containing 5cm3 water
3) Place each test tube in a water bath at a different temperature, for the same length of time
4) Remove the beetroot from the tubes with tweezers, leaving just the coloured liquid
5) Calibrate the colorimeter using a cuvette containing distilled water
6) Transfer a sample of the solution from the first test tube to a cuvette and record the absorbance of the solution using the colorimeter
- repeat for each solution
7) The higher the absorbance, the more pigment is release, so the higher the permeability of the membrane
RP5 is literally just cut open an organ system and write down what you see
Well done if you turned over this flashcard, clearly you are eager to learn more
Outline a method for investigating the effect of antimicrobial substances on microbrial growth (RP6)
1) Use a sterile pipette to transfer bacteria from the broth to an agar plate
- spread the bacteria over the plate using a sterile plastic spreader
2) Use sterile forceps to place paper discs soaked with different antibiotics spaced apart on the plate
- also add a negative control disk soaked in sterile water
3) Tape kid onto Petri dish, invert it and incubate at 25°C for 48 hours
- allows bacteria to grow, but prevents growth of pathogens
4) Size of inhibition zone indicates effectiveness of antibiotic
Give 5 aseptic techniques
1) Disinfect work surfaces to minimise contamination
2) Use sterile equipment or sterilise equipment independently by using Bunsen burner flame
3) Work near a Bunsen Bruner flame
- convection currents prevent microbes from contaminating plate
4) Minimise time spent with lid off agar plate
- or hold lid at 45° angle when spreading broth
5) Flame neck of broth container after opening and just before closing