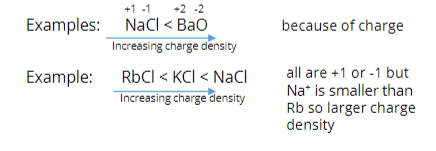On Ramps Chemistry ET: 24
1/3
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
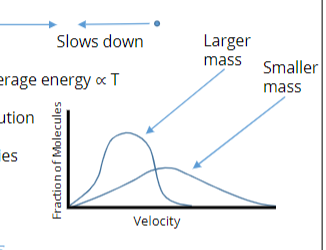
Maxwell Distribution
A collection of gas molecules undergoes collisions that can speed up or slow down velocity.
Result is a distribution with average energy ∝ T
As average T increases, distribution
spreads to higher velocity
As mass decreases, the velocities
spread
Physical Property Distribution
Know the following simple facts about liquid properties
• Evaporation – surface phenomenon in which the IMF of surface
molecules is broken by T to convert liquid to vapor
• Surface tension – surface phenomenon in which molecule IMF
makes molecules assume smallest surface area possible
• Boiling point – T at which molecules in the bulk achieve a vapor
pressure greater than atm pressure and escape
• Capillary action – tendency of surface molecules to adhere to
solid material surrounding the liquid
Assigning types of Solids
There are 4 types of solids:
• Metals – found on left side of periodic table (ex. Na, Fe, Au, Pb)
• Ionic – salts formed by cations from left side of table and anions on
right side of table (ex. NaCl, BaCl2, Al2O3)
• Network covalent – long chain of atom on right side of table (ex.
Wood, plastics, graphite, diamond)
• Molecular solids – small molecules you drew in Unit 2 that form IMF
at low T (ex. Ice, dry ice)
Ranking properties of solids (melting point)
Salts have boiling points that generally scale with charge density (similar to ranking lattice energies)