H Chemistry - Unit 2 - 2b - Alcohols
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
What is an alcohol?
An alcohol is a molecule containing a hydroxyl functional group.
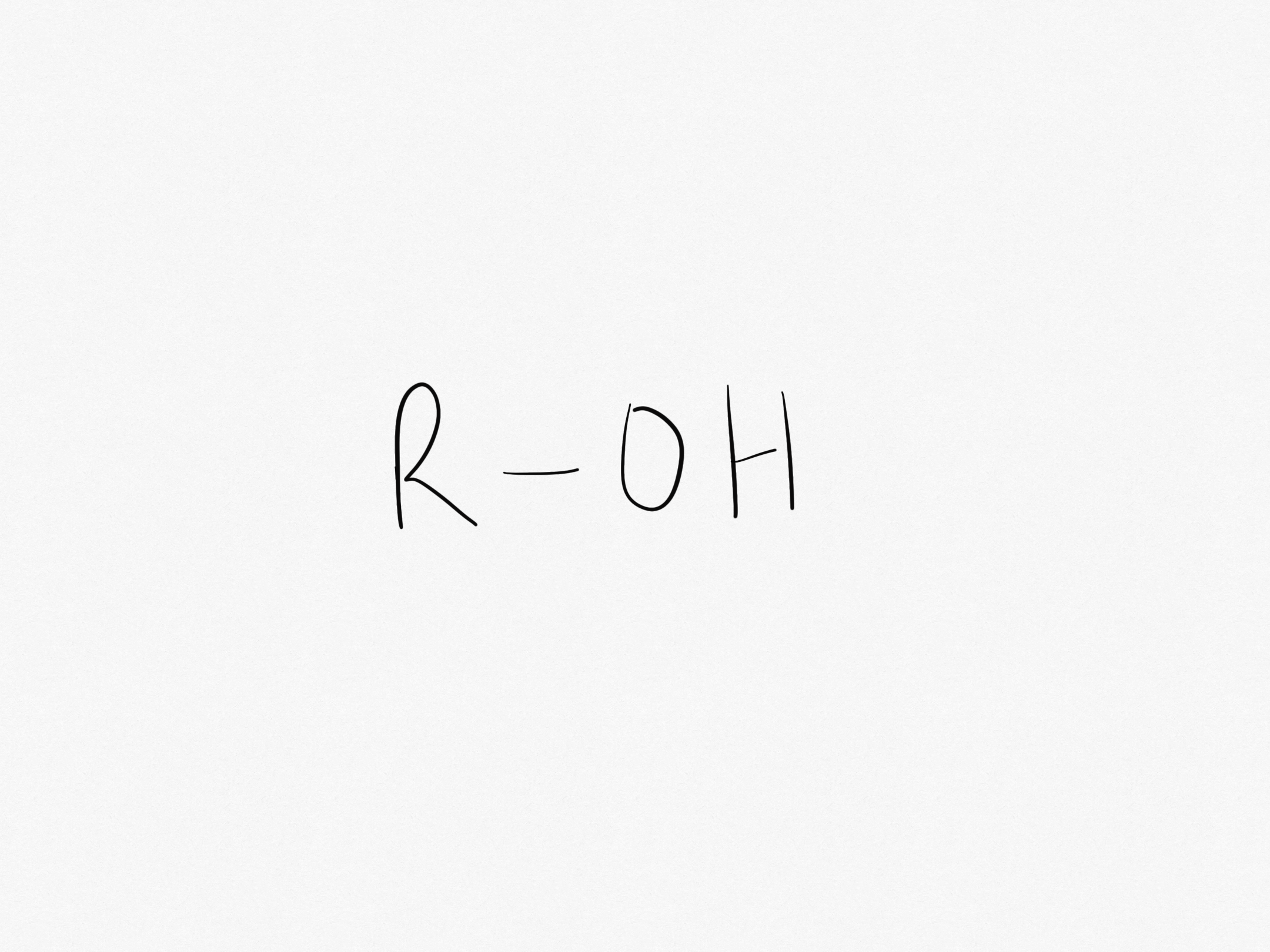
What is the hydroxyl functional group?
The hydroxyl functional group is -OH
What are the three classifications of alcohols?
The three classifications of alcohols are;
Primary
Secondary
Tertiary
What is a primary alcohol?
A primary alcohol is an alcohol that’s hydroxyl group is attached to a carbon that is attached to 1 or 0 other carbons.
What is a secondary alcohol?
A secondary alcohol is an alcohol that’s hydroxyl group is attached to a carbon that is attached to two other carbons.
What is a tertiary alcohol?
A tertiary alcohol is an alcohol that’s hydroxyl group is attached to a carbon that is attached to three other carbons.
What are alcohols containing two hydroxyl groups known as?
Alcohols with two hydroxyl groups are called diols.
What are alcohols containing three hydroxyl groups known as?
Alcohols with three hydroxyl groups are called triols.
What characteristic do hydroxyl groups give alcohols?
Hydroxyl groups make alcohols polar and this gives rise to hydrogen bonding.