Junior Cert Revision
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
What is an element?
Matter made up one type of atom only that can’t be split into anything simpler by chemical means
What is a compound?
Matter made up of 2 or more different types of elements chemically combined
What is a molecule?
Combination of 2 or more atoms chemically bonded together
What are the two types of molecules
Molecule of an element, molecule of a compound
What is a molecule of an element
2 or more of the same atoms chemically combined
What is a molecule of a compound
2 or more different atoms chemically combined
True or false: All compounds are molecules
True
True or false: All molecules are compounds
False
What are diatomic molecules?
A group of elements that exist in nature not as atoms, but as 2 atoms of the same element bonded to each other
What are the elements that are diatomic molecules?
Hydrogen, Nitrogen, Fluorine, Oxygen, Iodine, Chlorine, Bromine

What does this diagram show?
Atom of an element
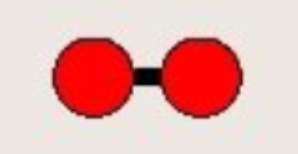
What does this diagram show?
Molecule of an element
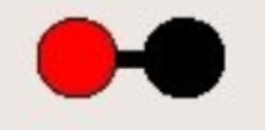
What does this diagram show?
Molecule of a compound
What is a mixture
2 or more different particles mingled together, but not chemically combined
True or false: mixtures have distinct properties
False
True or false: Mixtures are the same sample to sample
False: Mixtures vary from sample to sample
What are the two different types of mixtures
Homogeneous and heterogeneous
What is a heterogeneous mixture
A mixture that is not the same throughout
What is a homogeneous mixture
A mixture that is the same throughout
What does filtration do
Separate insoluble solids from liquids
What are the two types of filtration
Gravity and suction/vacuum
What is gravity filtration
Uses gravity to pull water through filter paper
What is suction/vacuum filtration
Uses a buchner flask as opposed to a conical flask. Faster than gravity filtration
What is the name of the solid separated from the liquid during filtration
Residue
What is the name of the liquid separated from the solid in filtration
Filtrate
What is evaportation
Separate a soluble solid from a liquid by evaporating the liquid, leaving the solid behind
What is distillation
Separates components of a mixture based on their boiling points
What are the steps to distillation
In the distilling flask, mixture heated & liquid with lower boiling point evaporated
Use thermometer to monitor temperature
Liebig condenser cools vapour to condense it
Receiving flask collects distillate (evaporated, cooked and condensed liquid)