CHEM All Lesson Prelim G12 Part 1
1/85
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
86 Terms
Kinetic Molecular Theory
A model used to explain the behavior of matter. Matter is composed of tiny particles that are always in motion.
Kinetic Energy
Matter is made of particles that are constantly in motion. This energy in motion is called ______________

Temperature
The amount of kinetic energy in a substance is related to its ______________

Greater speed
Increased temperature means _____________ ___________
Space
There is _______ between particles. The amount of ________ between particles is related to the substance's state of matter.
Phase changes
_________ _________ happens when the temperature of the substance changes sufficiently.
intermolecular forces
There are attractive forces between the particles called __________________ ____________
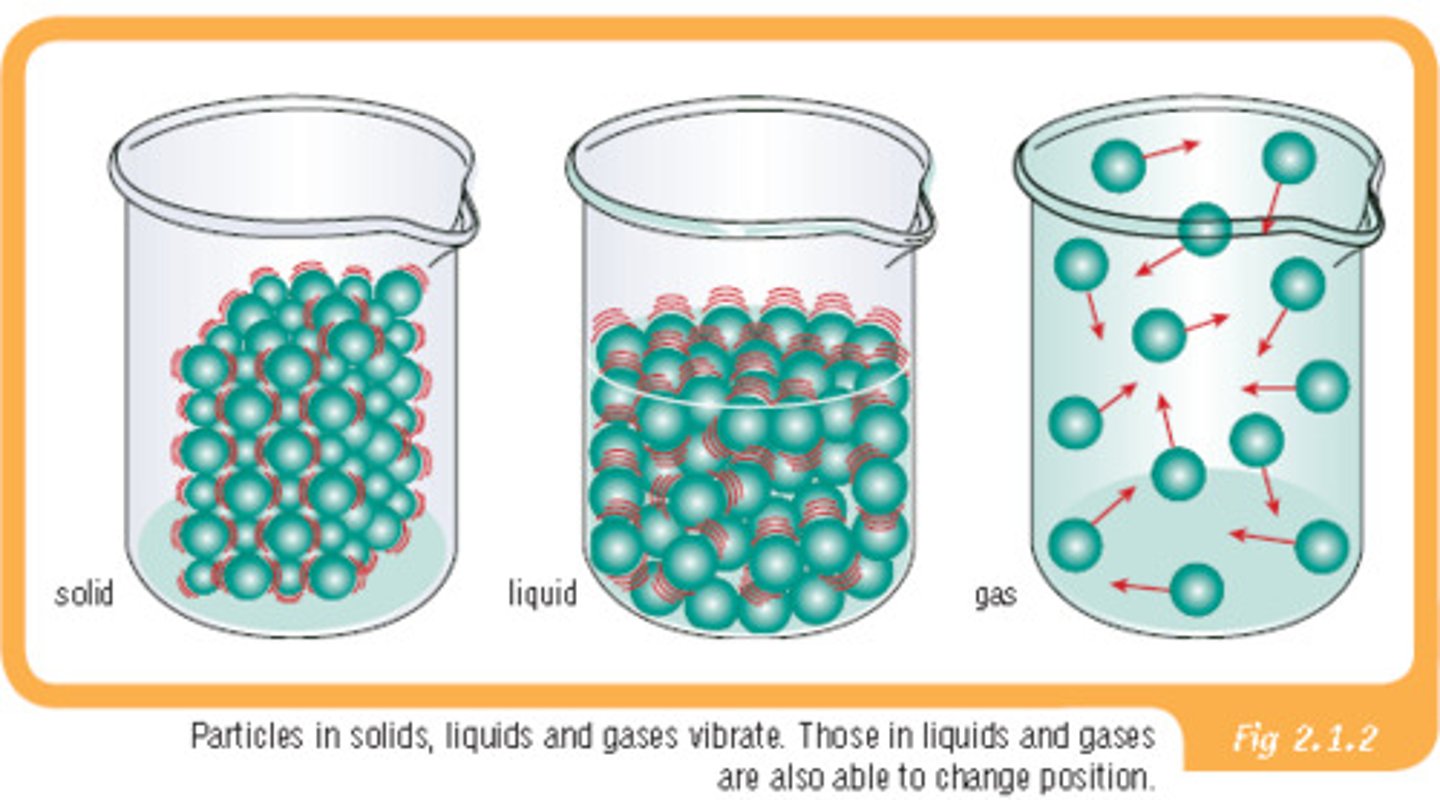
Solid
Particles are tightly packed and has a fixed shape and volume.
Liquid
Particles are close but can move and has a fixed volume but no fixed shape.
Gas
Particles are far apart and move freely and no fixed shape or volume.
Chemical Bonding
Forces that holds atoms together.
Molecules
A group of atoms bonded together, representing the smallest fundamental unit of a chemical compound. Made up of two or more atoms. The atoms can be the same or different.
Compounds
Formed when different elements chemically react together (bond)
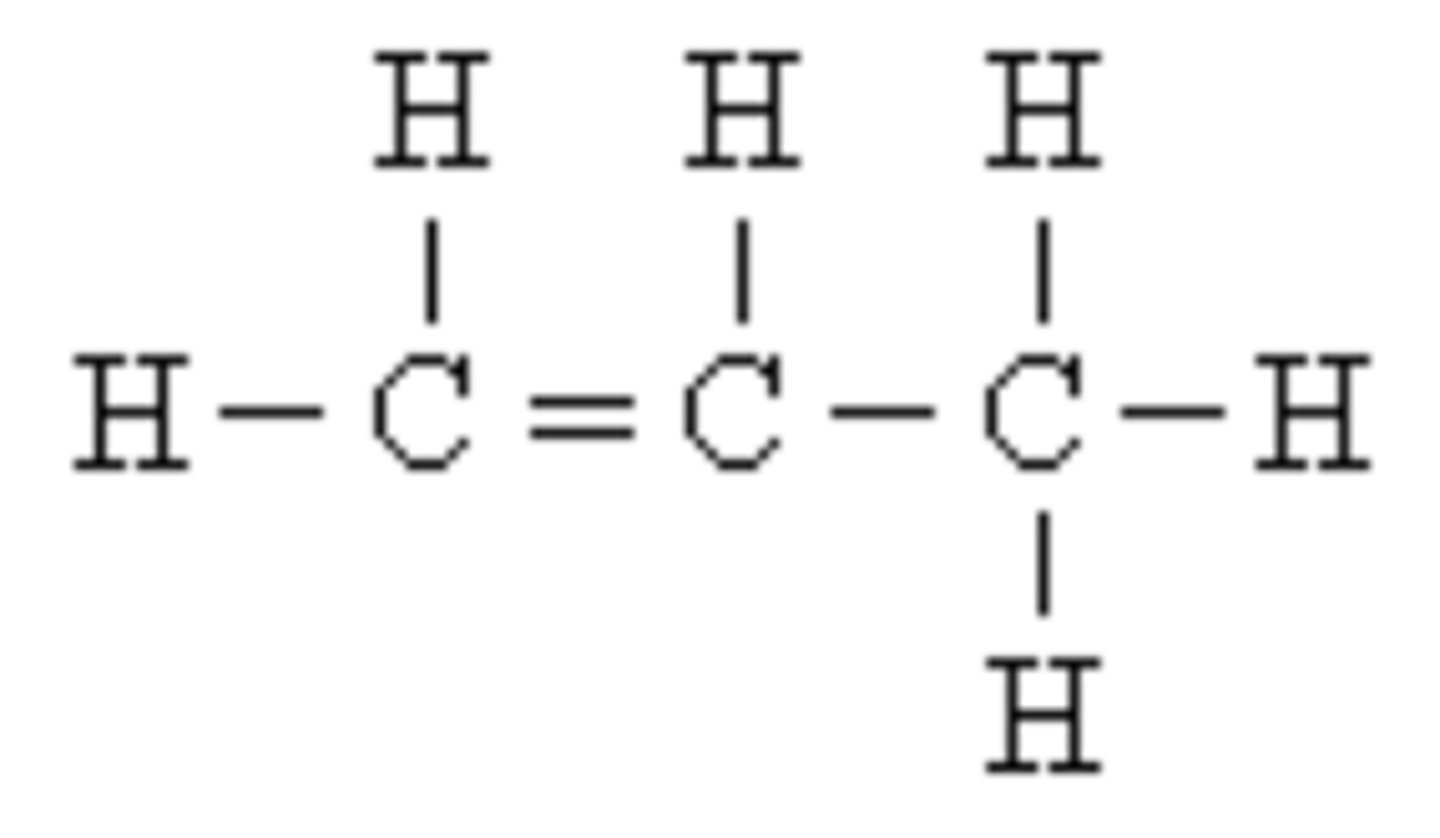
Valence Electrons
Electrons in the outermost orbital. Create unbalanced charges.Cause atoms to be reactive and unstable. Bonds form because atoms are seeking stability.
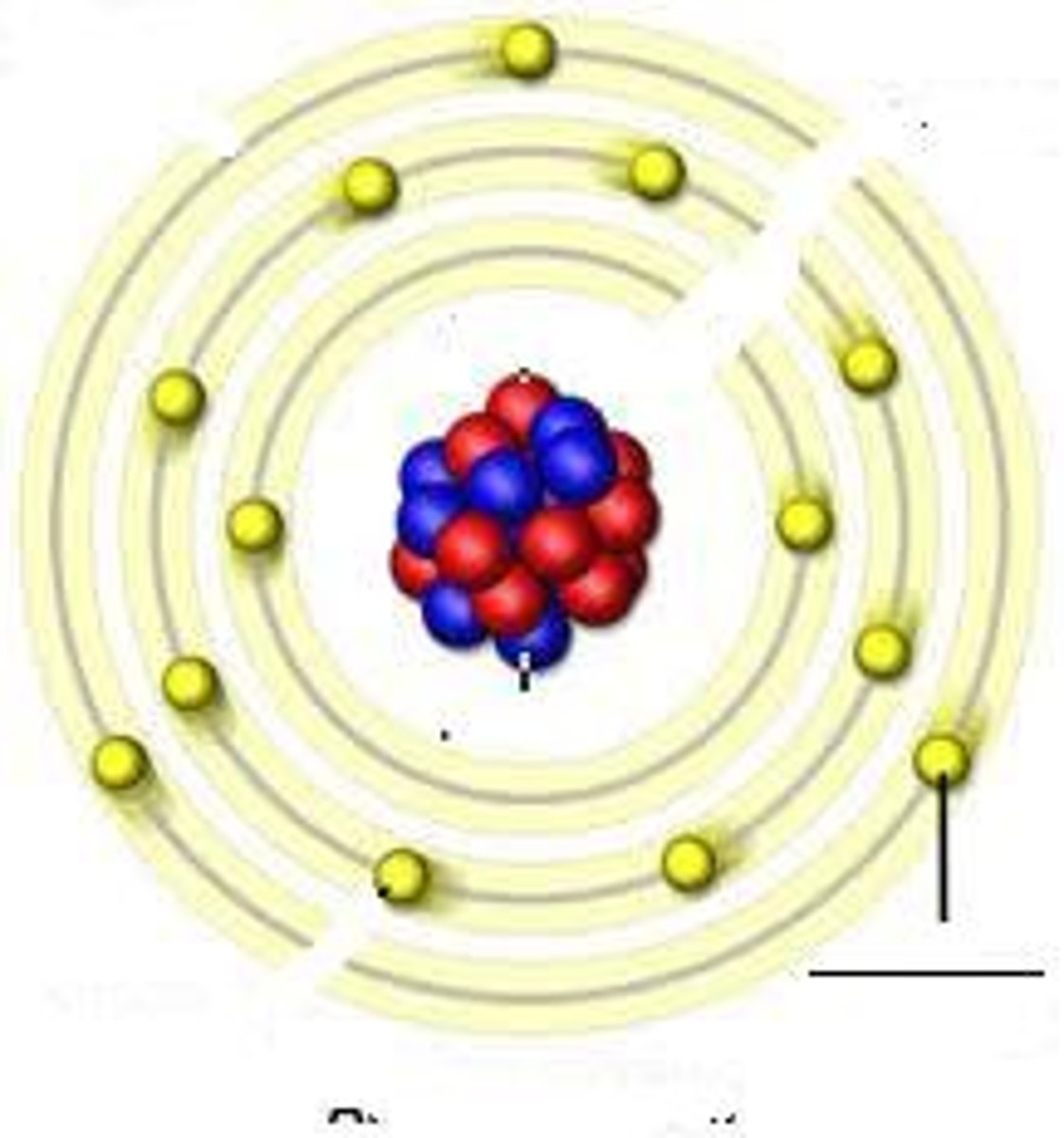
Energy Levels
Layers in the electron cloud where electrons exist.
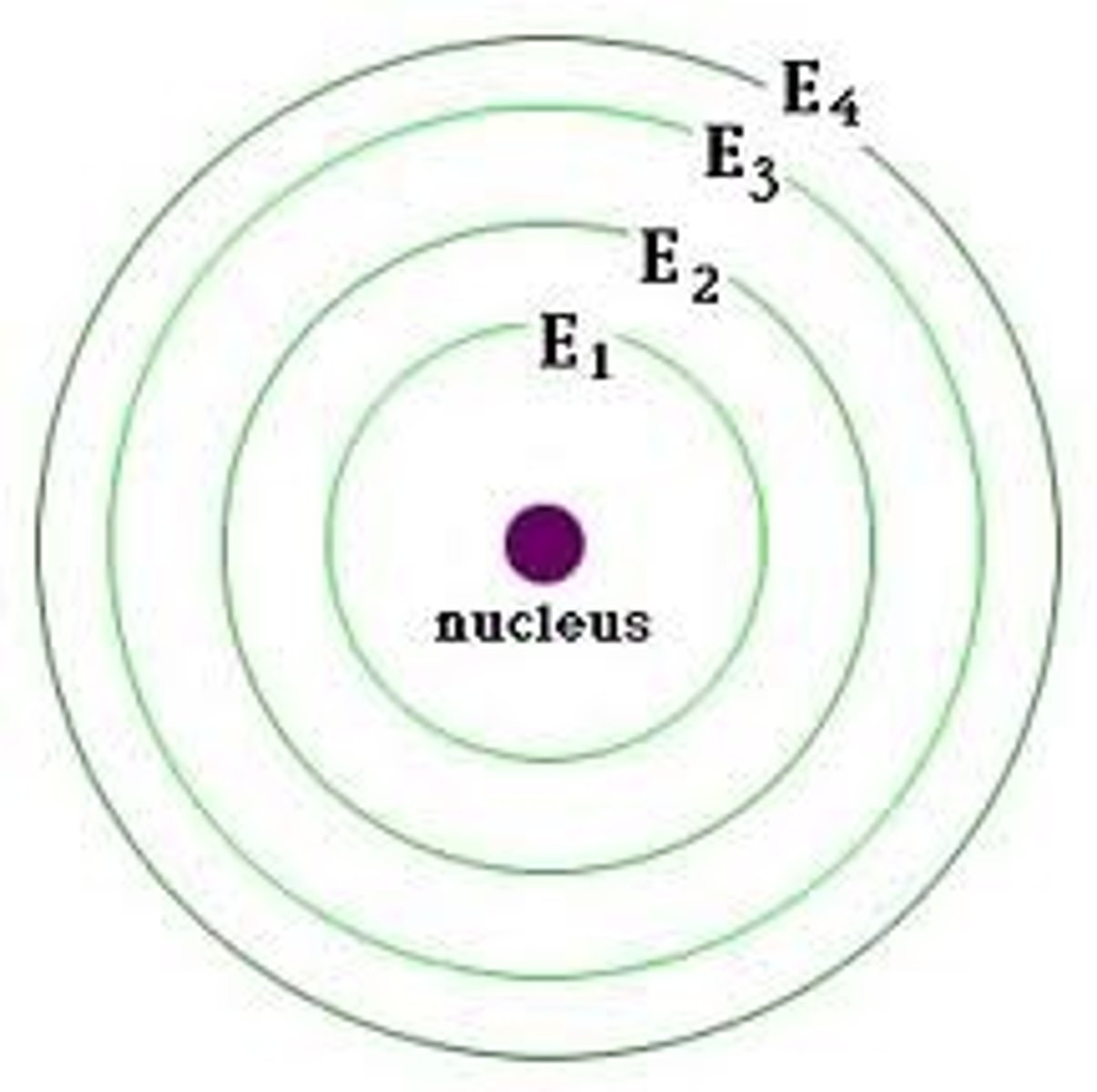
2 Electrons
Level 1 energy level
8 Electrons
Level 2 energy level
18 Electrons
Level 3 energy level
Valence energy level
Outer most level called the __________ __________ ________ which CANNOT hold more than 8 electrons.
Octet rule
An atom is most stable when there are eight (8) electrons in its outermost orbital. Chemical compounds tend to form so each atom (by gaining, losing, or sharing electrons) has 8 electrons in its outermost energy level. HAPPY ATOMS!
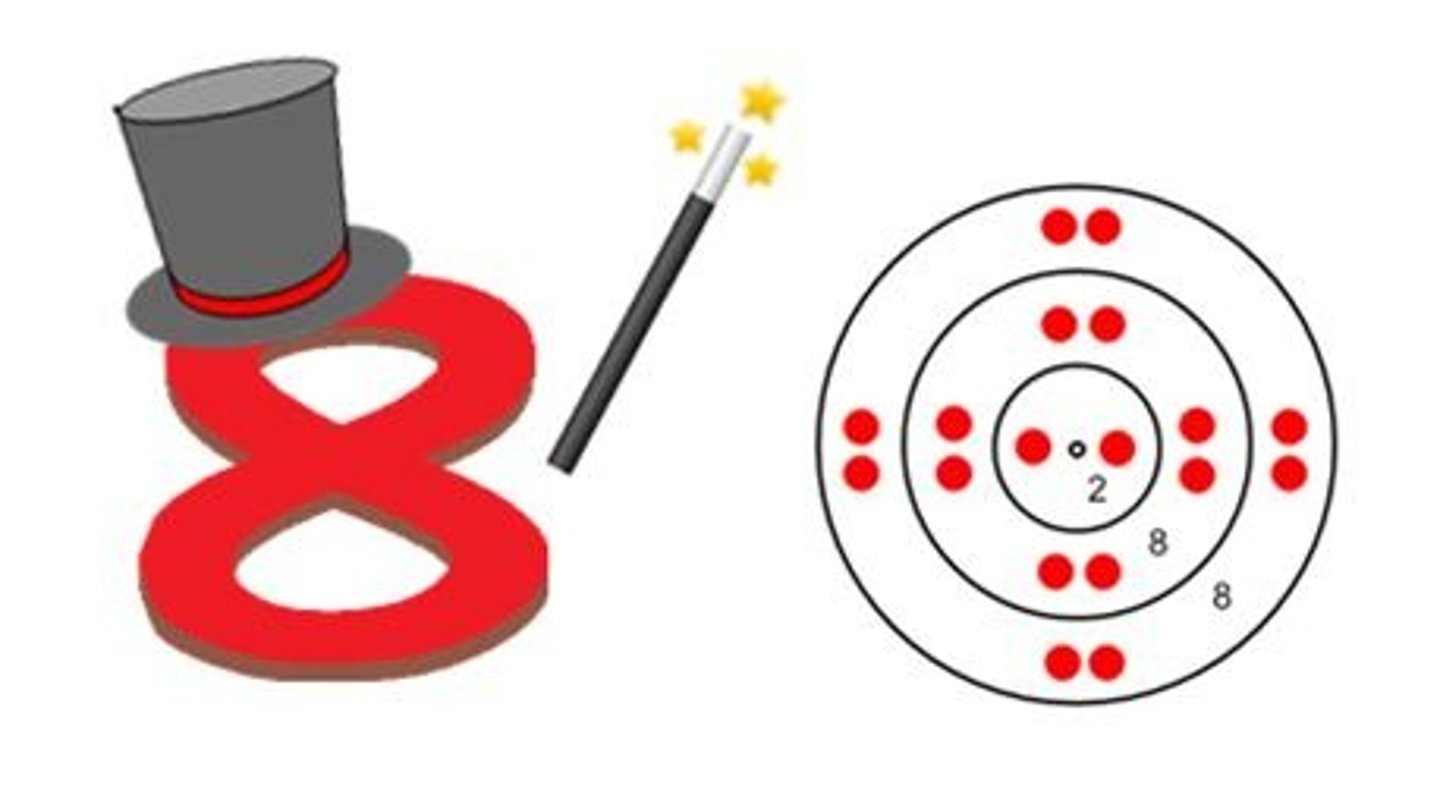
Stable atoms
Happy atoms are called ___________ ___________
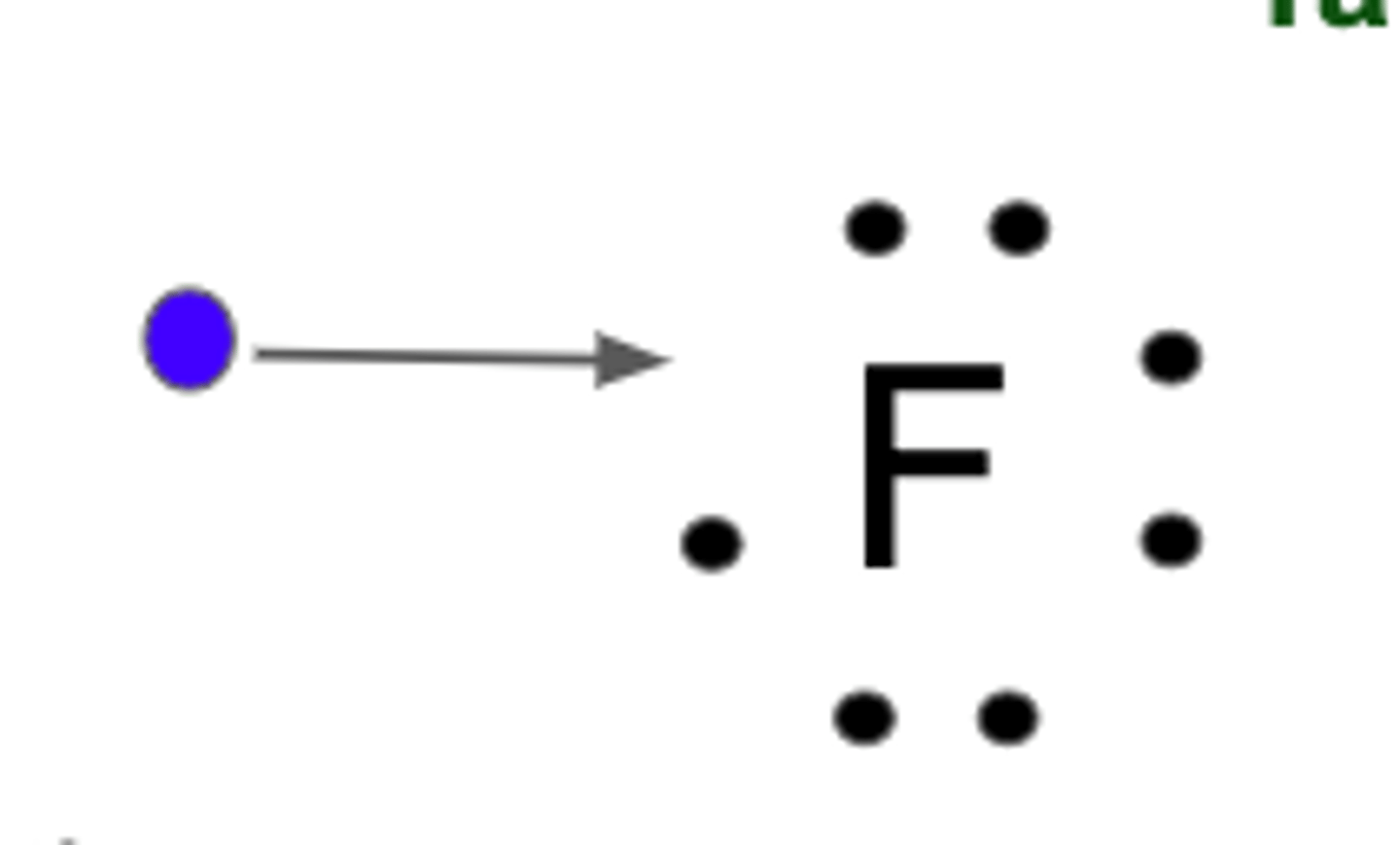
Ionic Bonds
Formed by transfer of electrons, formed between atoms of nonmetals and metals. Form ions (charged particle) Ions of opposite charge attract each other forming the bond (like a magnet, opposites attract).
Properties of Ionic Bonding
Form solids at ordinary temperatures. Organized in the characteristic crystal lattice of alternating positive and negative ions. Always exothermic. High melting and boiling point. Soluble in water.
Covalent Bonds
Formed by sharing of a pair of electrons, formed between atoms of nonmetals. Do not form ions when bonding. Happens in the outermost energy level with valences electrons.
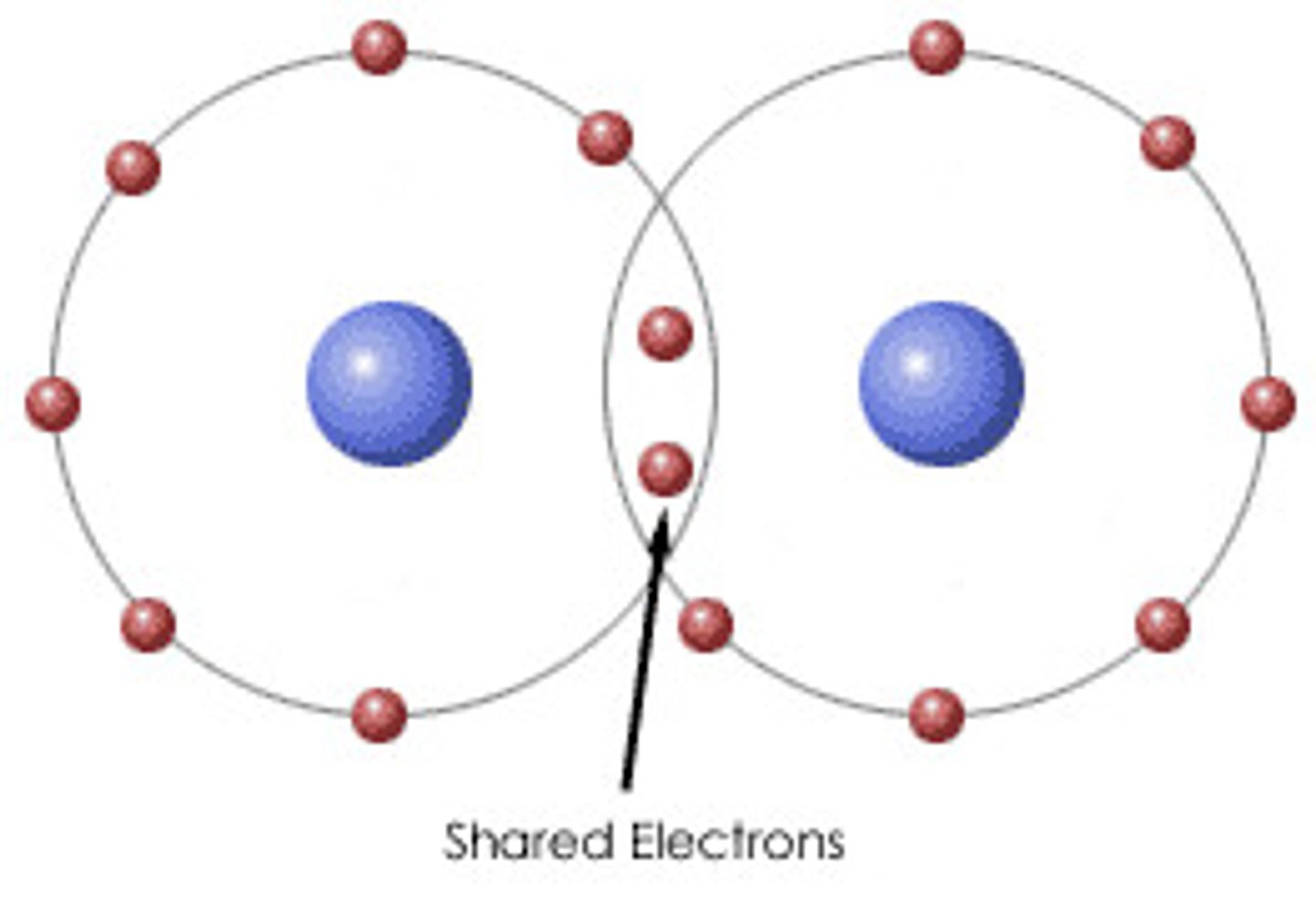
Properties of Covalent Bonding
Exist as gases, liquids, and solids. Bad conductors. Insoluble in polar solvents (water). Low melting and boiling points. Flammable and brittle.
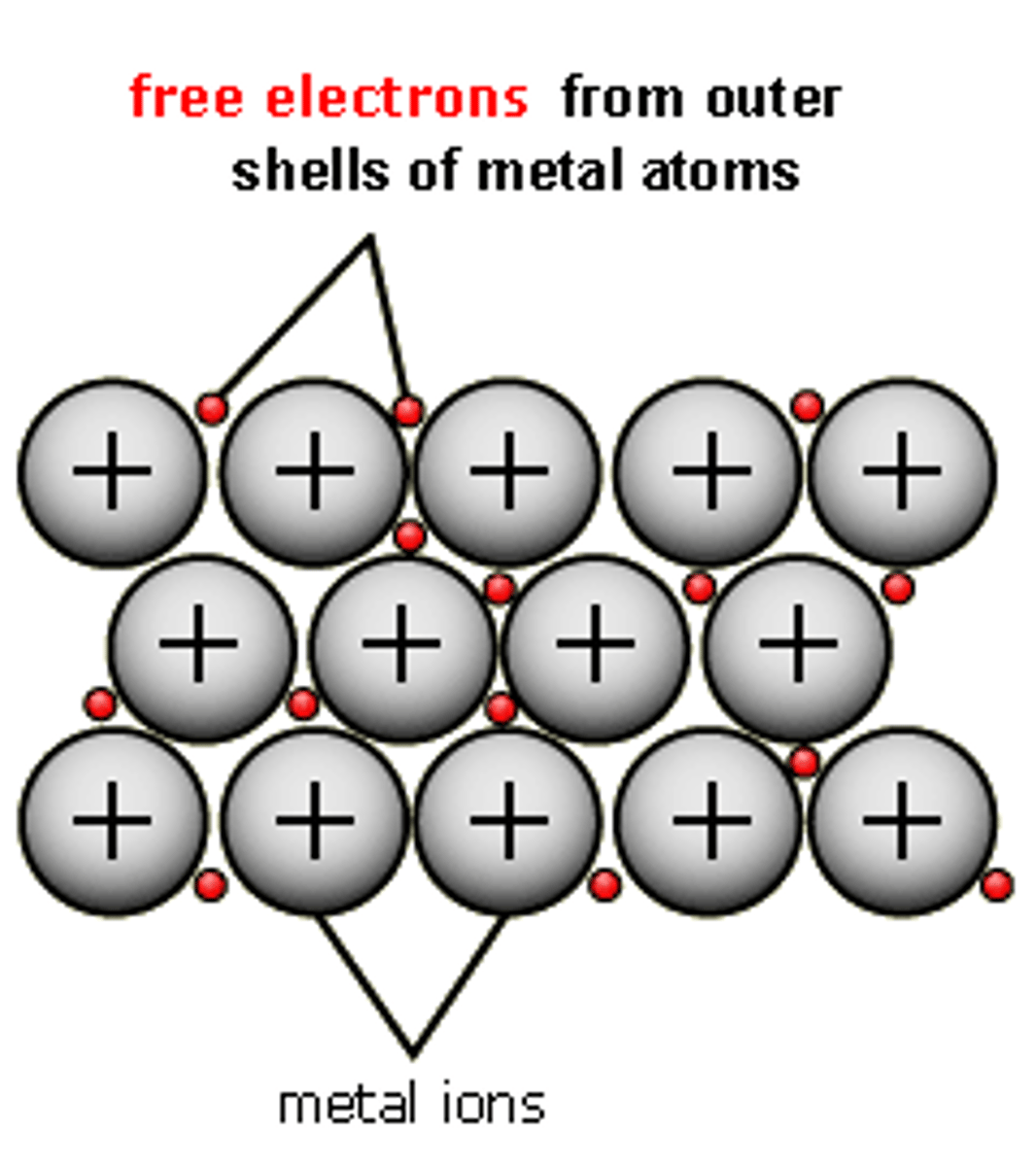
Metallic Bonds
Occurs between atoms of metals. Formed via attraction of the mobile electrons referred to as a sea of electrons and the fixed positively charged metal ions.
Intramolecular Force
Forces that holds atoms in a molecule. Stronger that intermolecular forces. Determine the chemical behavior of a substance. Chemical bonds. Categorized into Covalent, Ionic and Metallic bond.
Electronegativity of < 0.4
Non-polar Covalent Bond
Electronegativity of 0.4 - 1.7
Polar Covalent Bond
Electronegativity of > 1.7
Ionic Bond
Molecular Shape
The geometric shape formed by atoms bonded to the central atom in a molecule.
Electron domain geometry
Arrangement of electron domains in 3D space
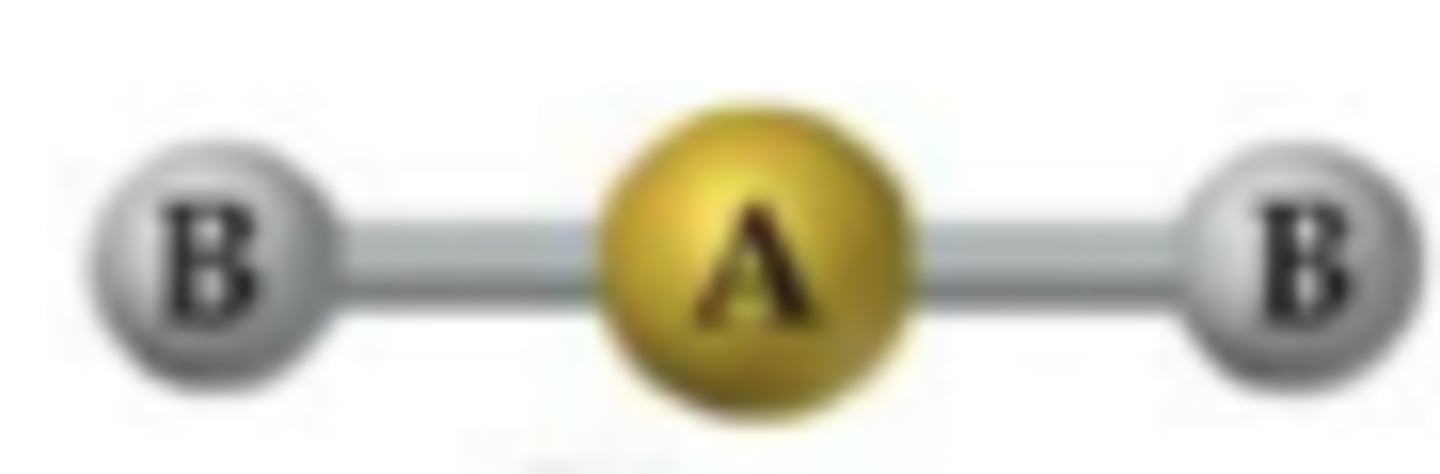
Molecular geometry
Arrangement of atoms in the molecule
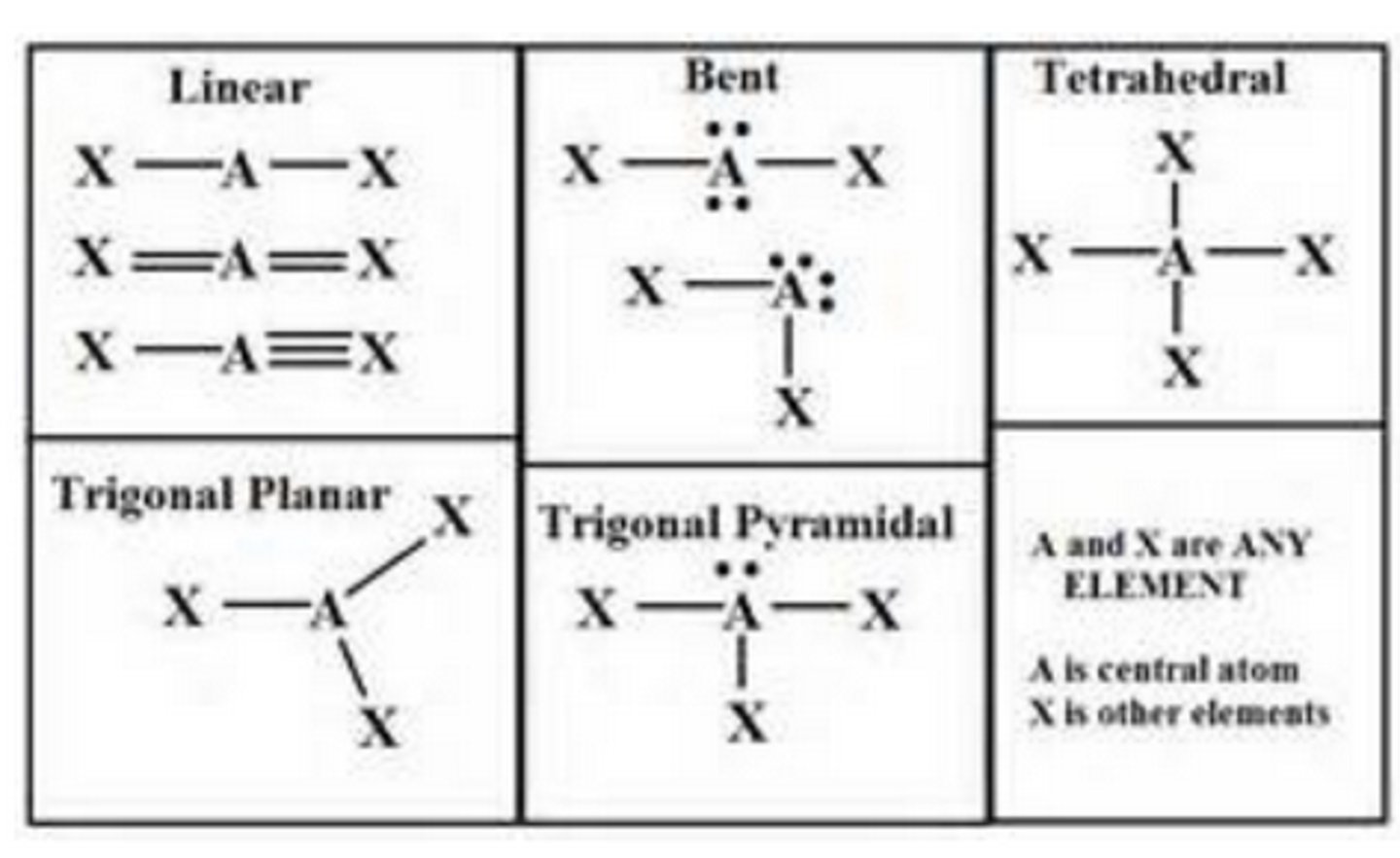
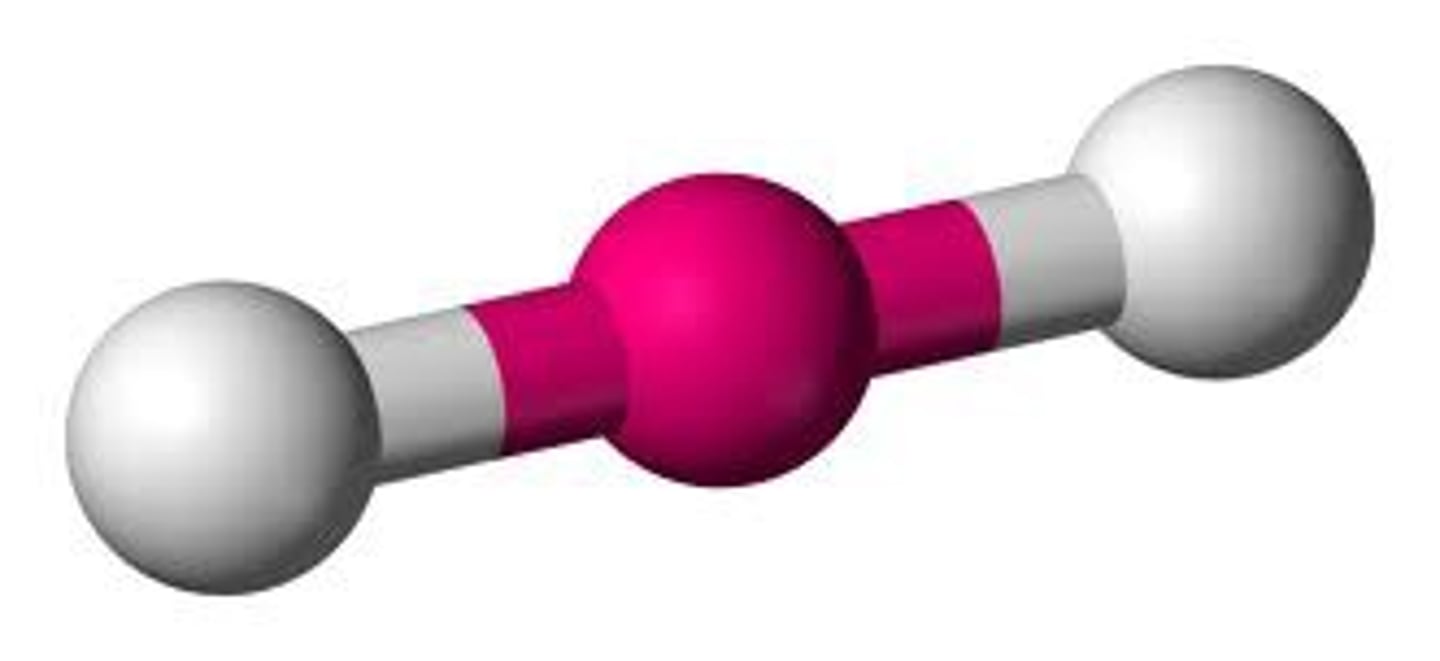
Linear
No. of Electron Domains 2
No. of Bonding Domains: 2
No. of Lone Pairs: 0
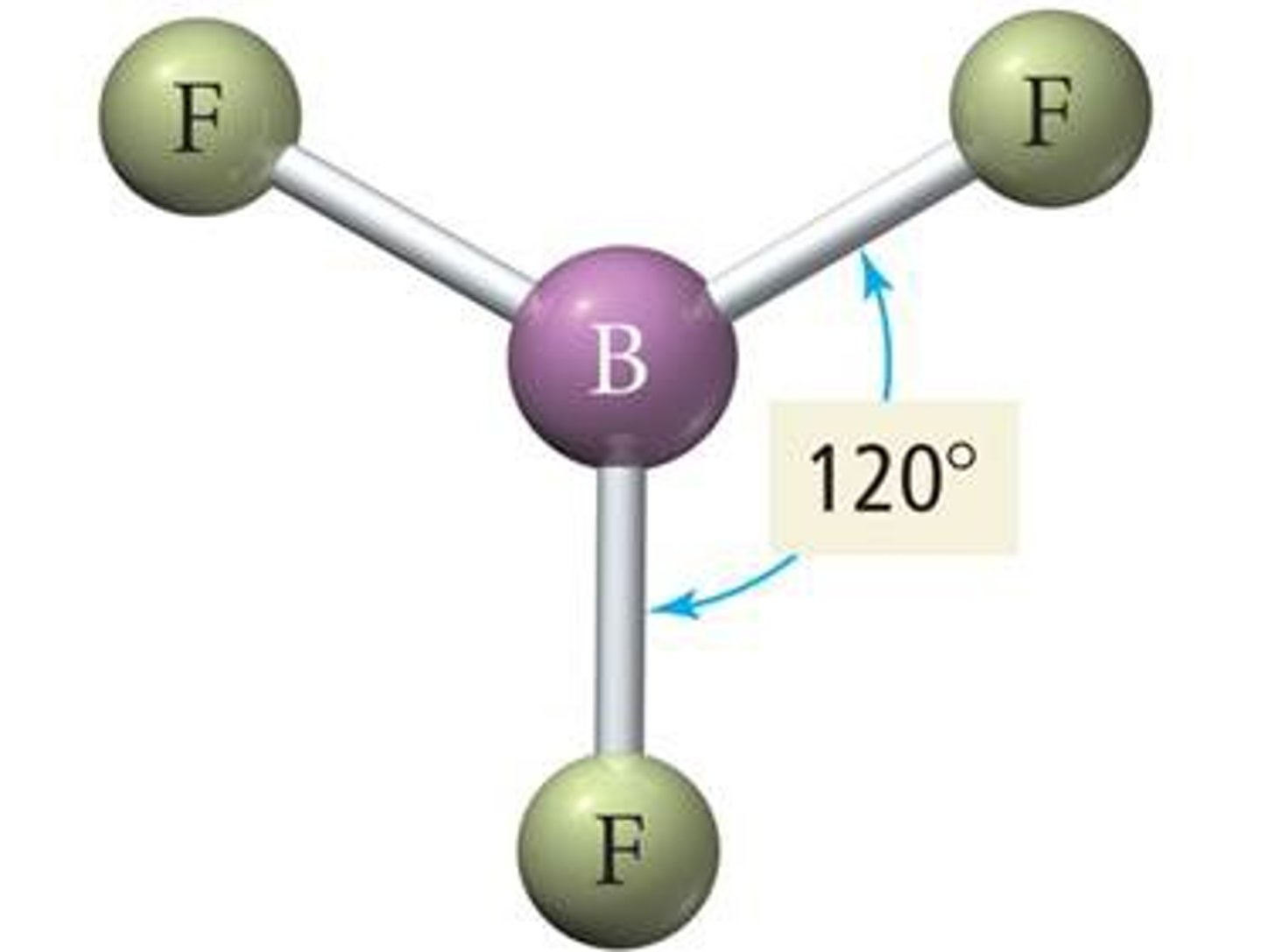
Trigonal Planar
No. of Electron Domains 3
No. of Bonding Domains: 3
No. of Lone Pairs: 0
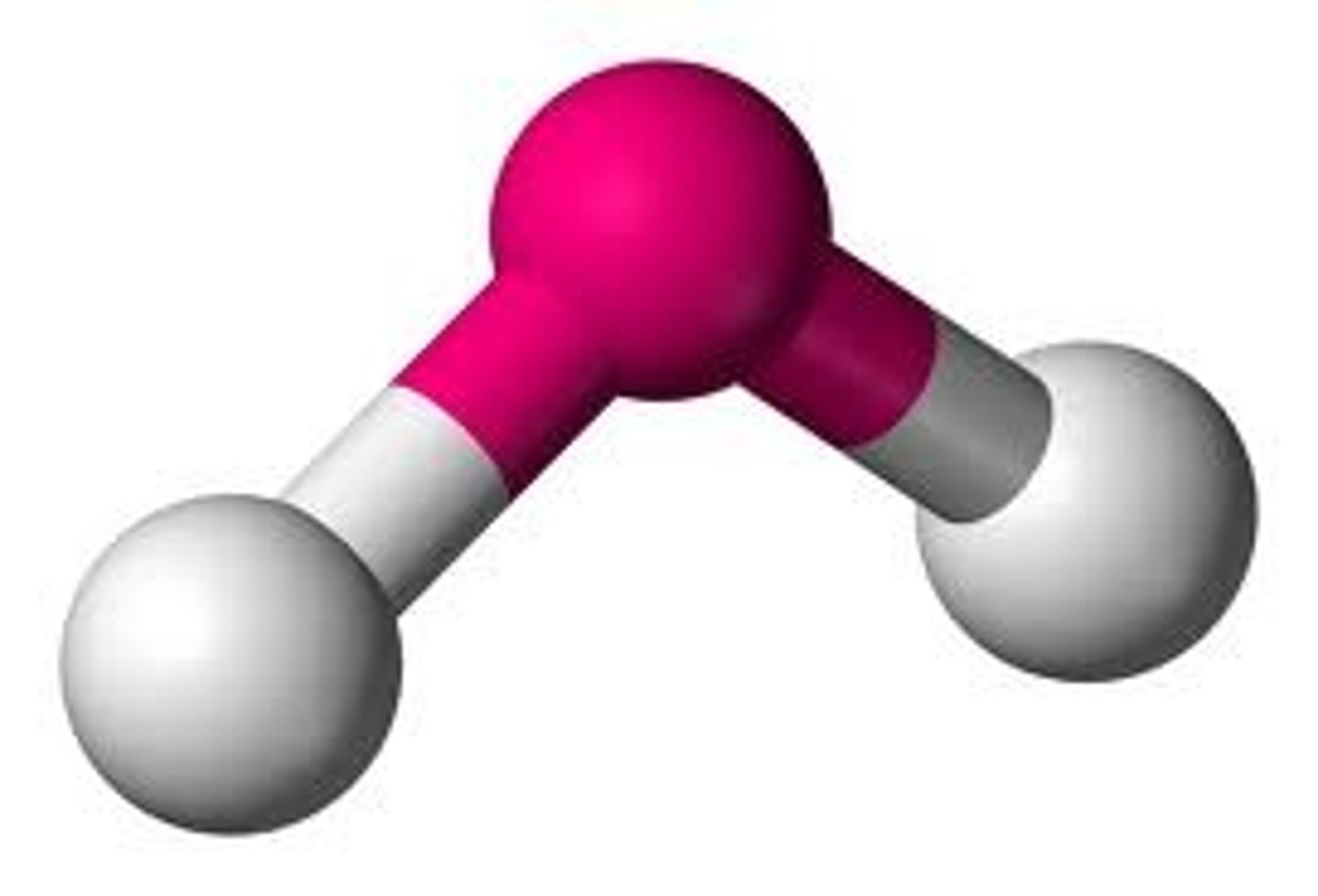
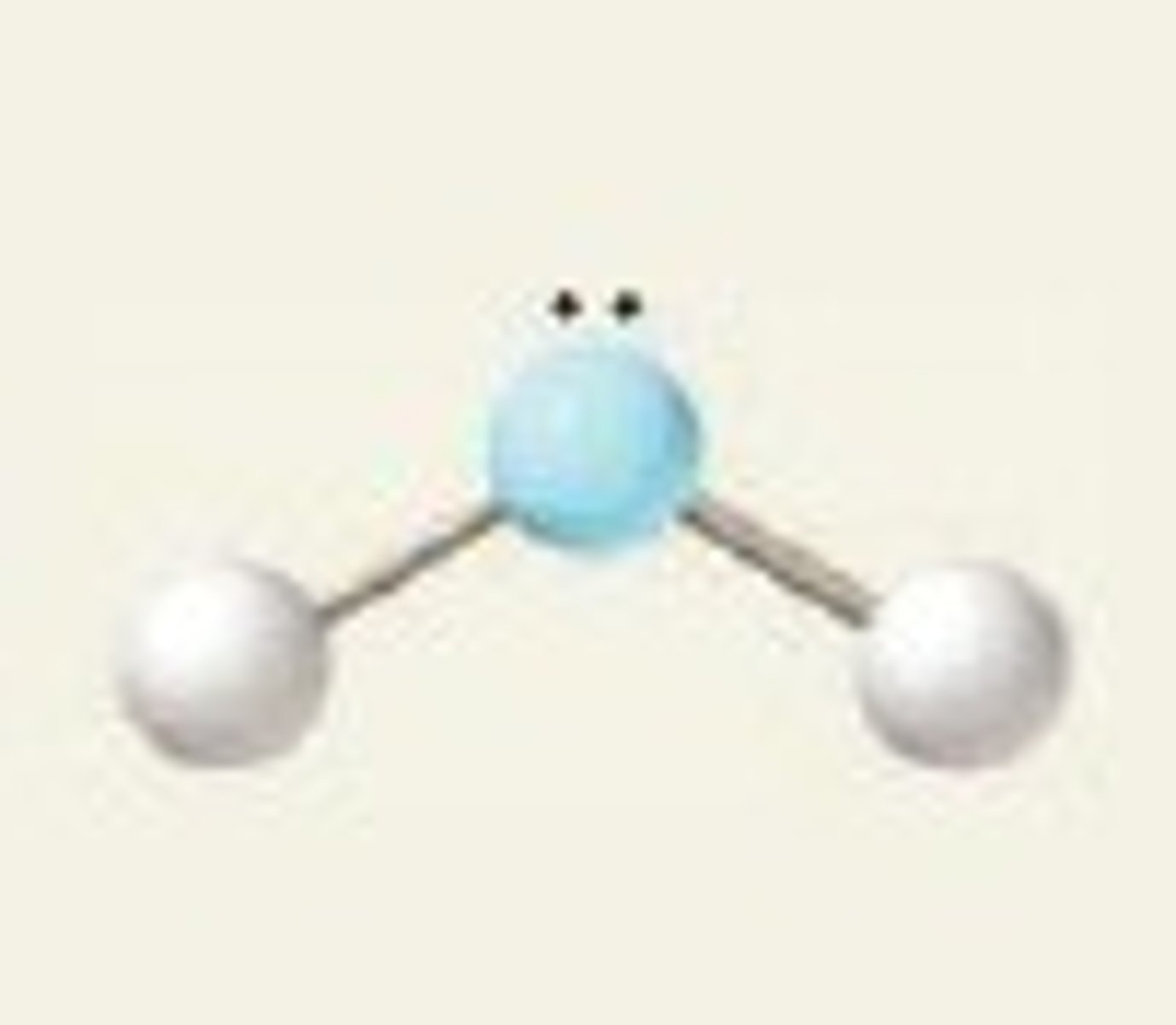
Bent
No. of Electron Domains 3
No. of Bonding Domains: 2
No. of Lone Pairs: 1
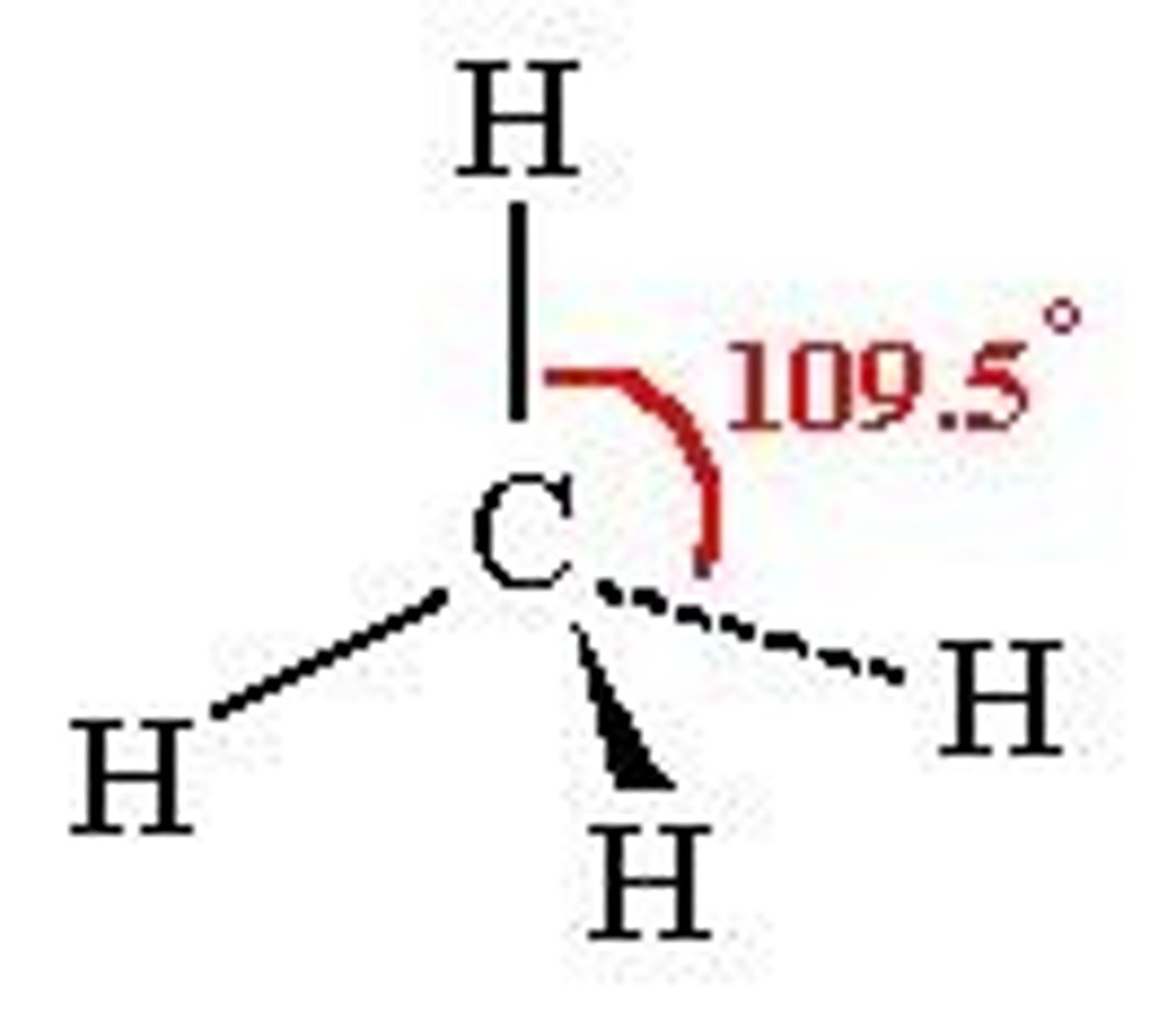
Tetrahedral
No. of Electron Domains 4
No. of Bonding Domains: 4
No. of Lone Pairs: 0
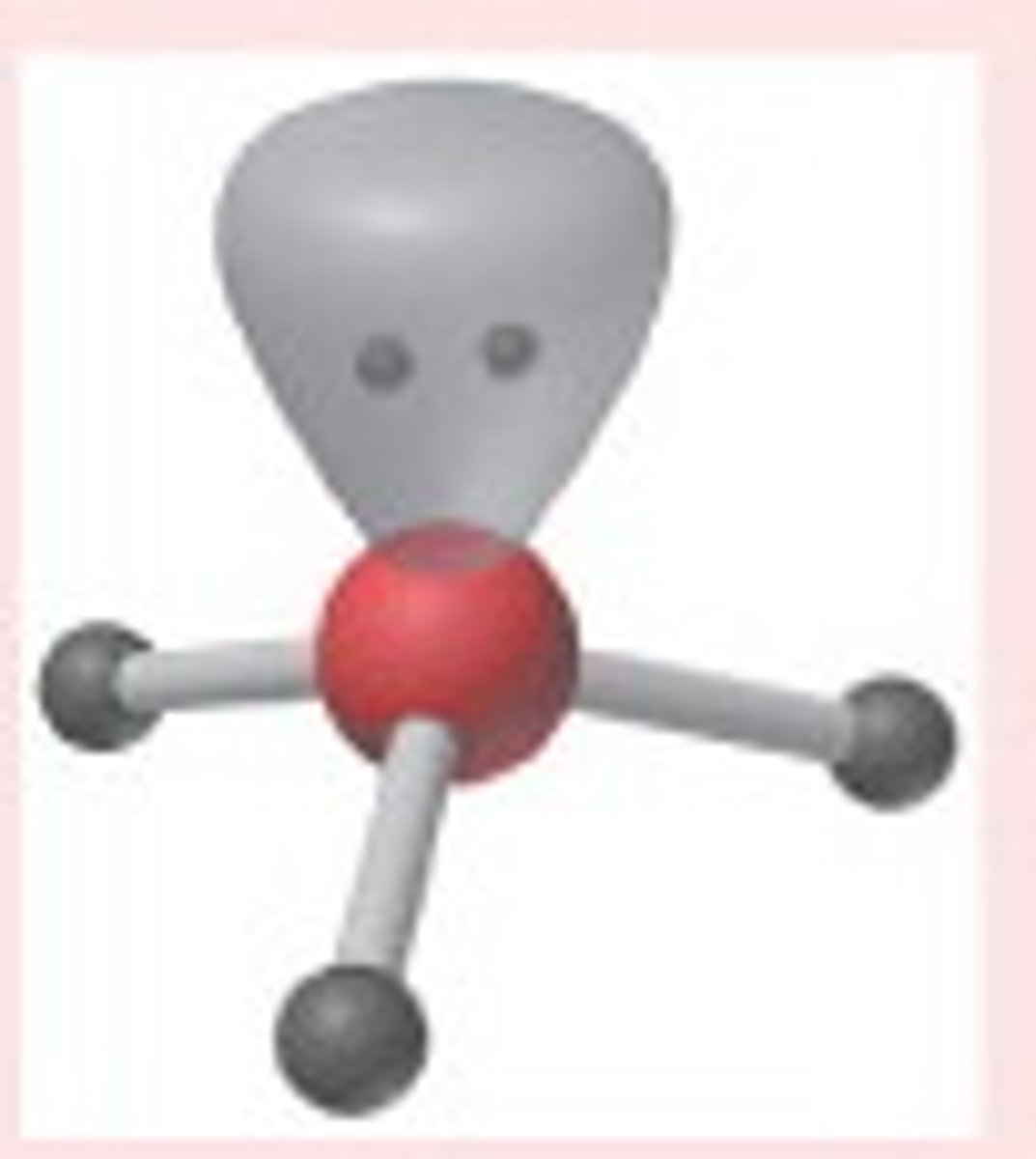
Trigonal pyramidal
No. of Electron Domains 4
No. of Bonding Domains: 3
No. of Lone Pairs: 1
Trigonal bipyramidal
No. of Electron Domains 5
No. of Bonding Domains: 5
No. of Lone Pairs: 0
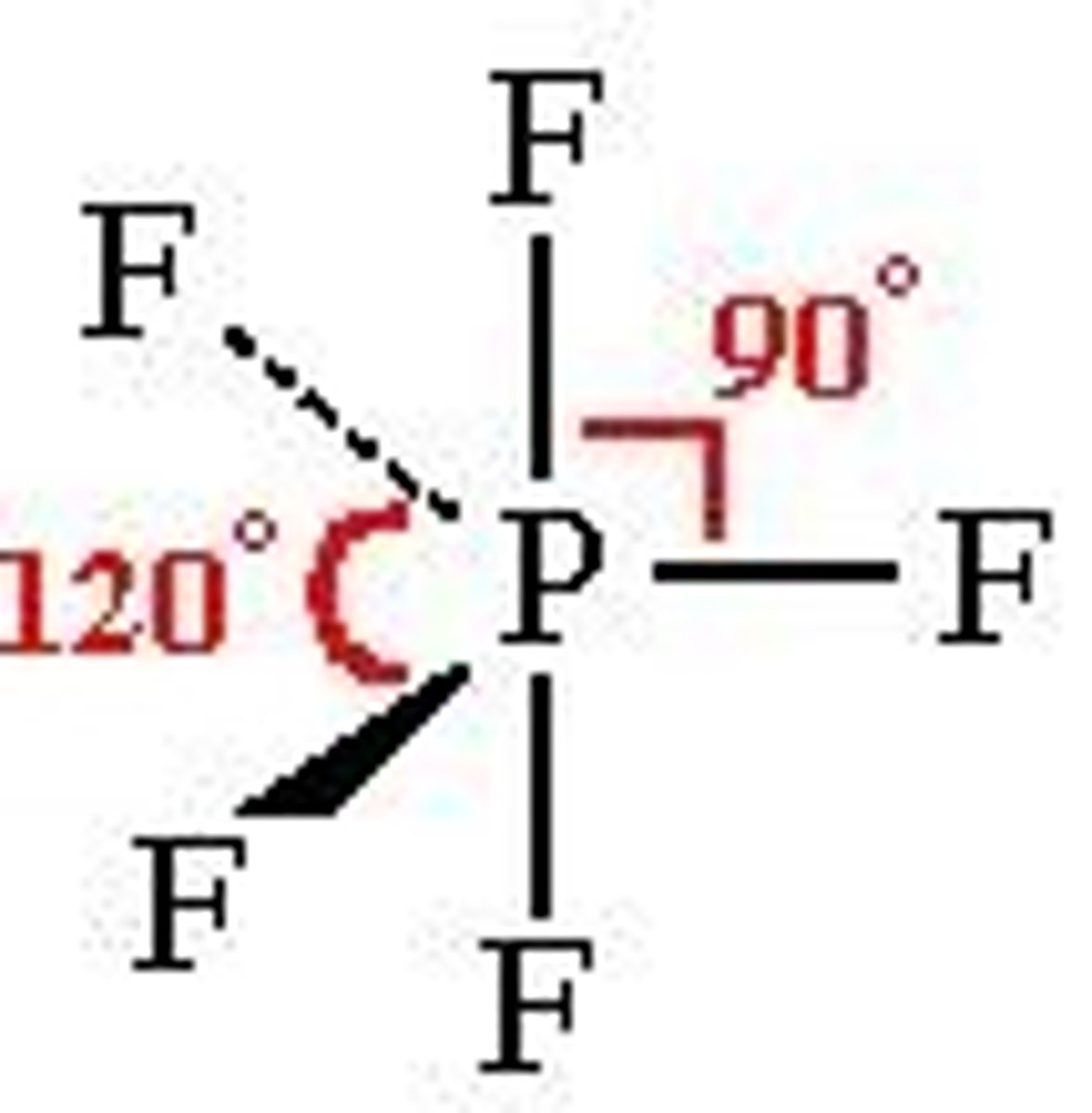
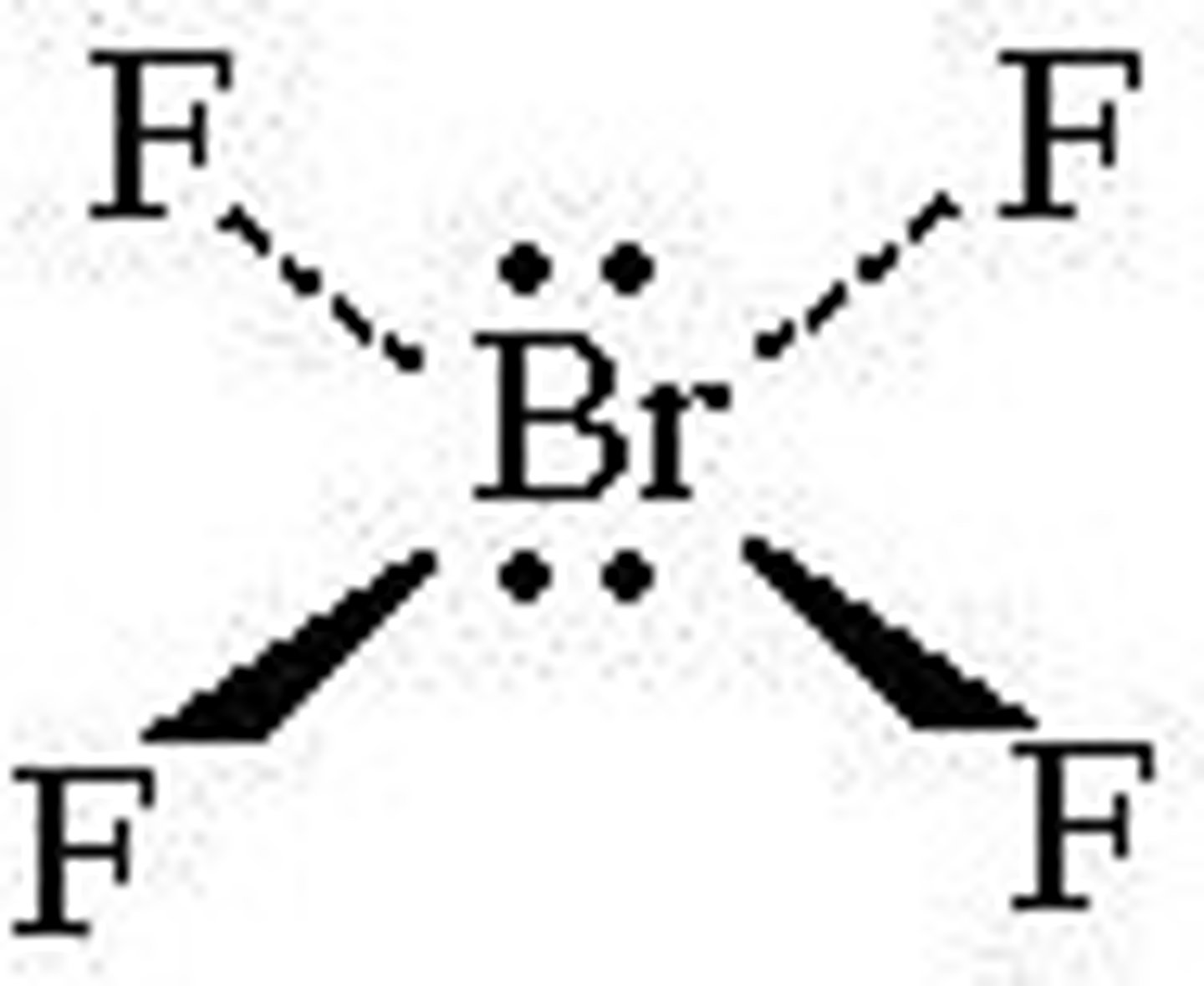
Seesaw
No. of Electron Domains 5
No. of Bonding Domains: 4
No. of Lone Pairs: 1
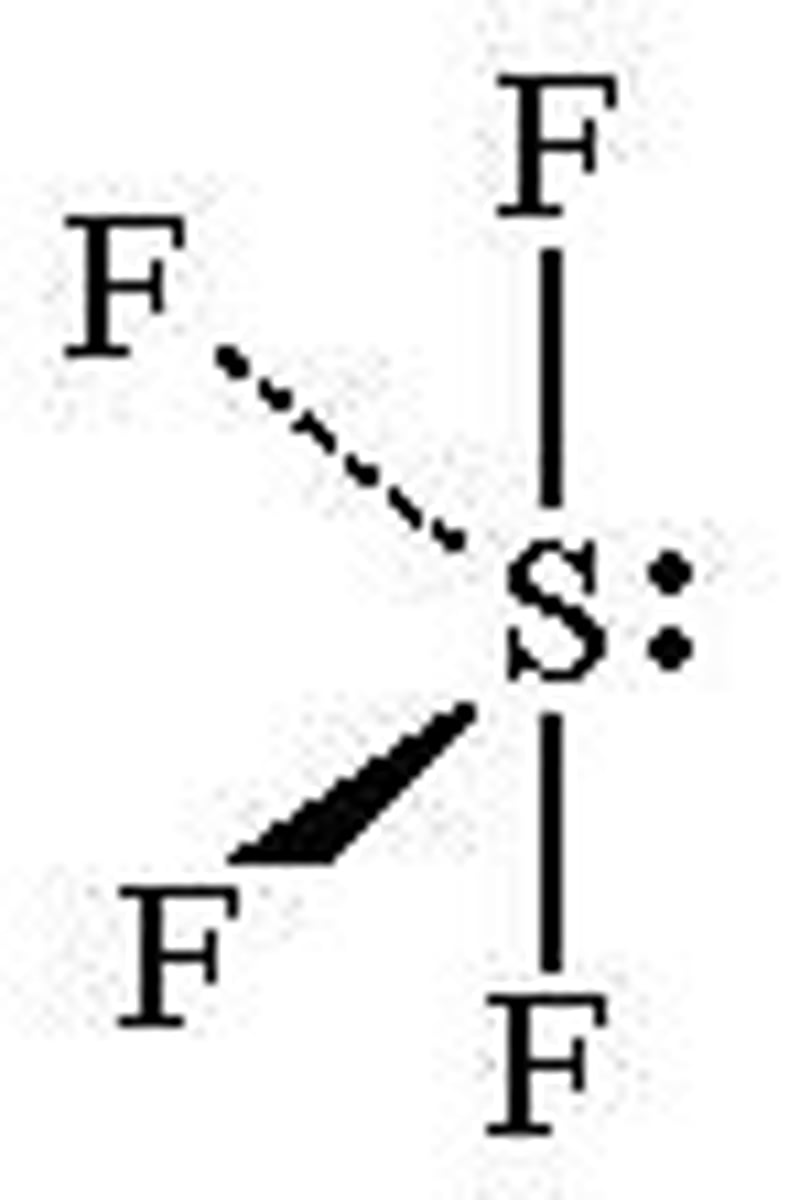
T-shaped
No. of Electron Domains 5
No. of Bonding Domains: 3
No. of Lone Pairs: 2
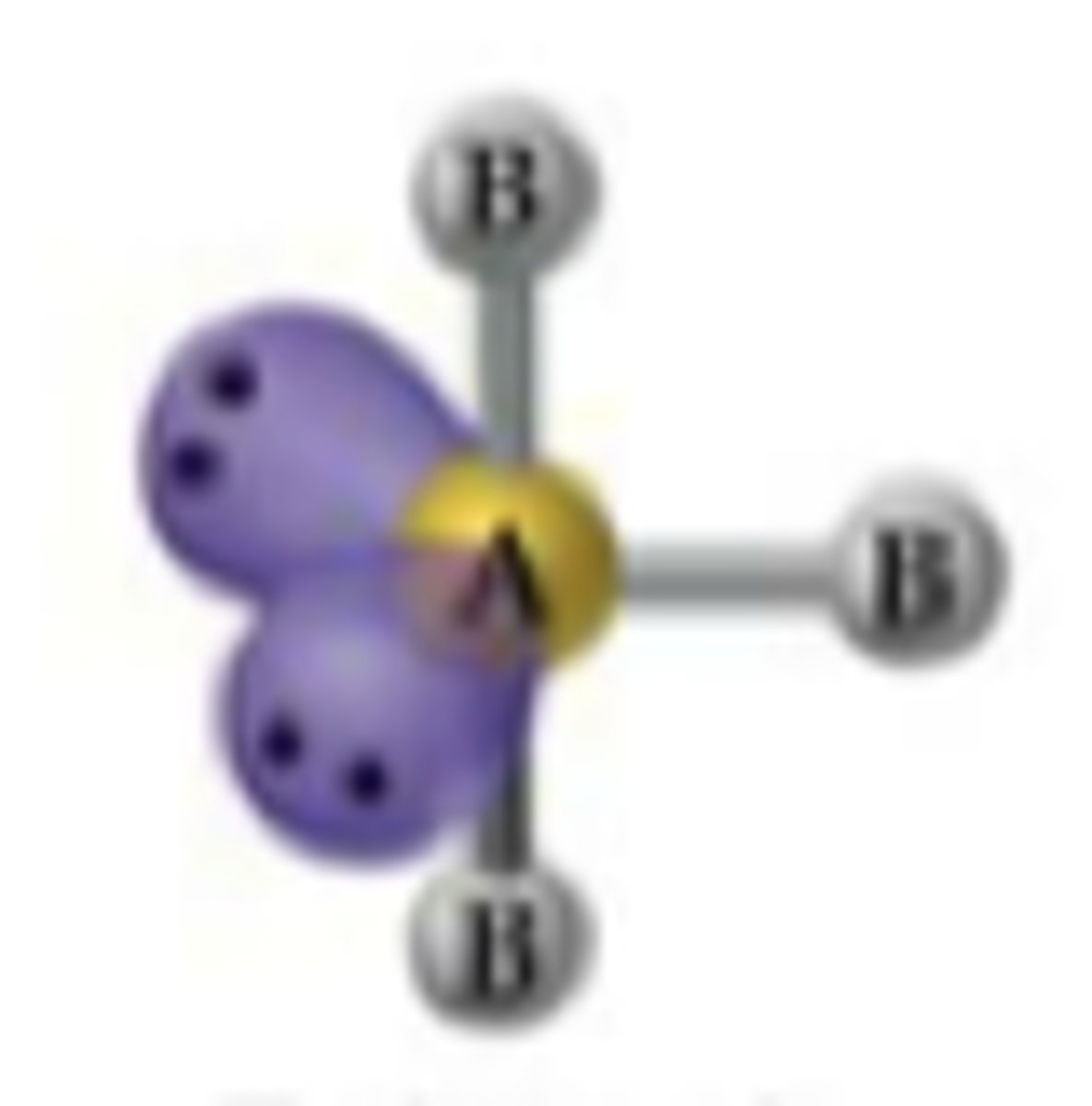
Linear
No. of Electron Domains 5
No. of Bonding Domains: 2
No. of Lone Pairs: 3
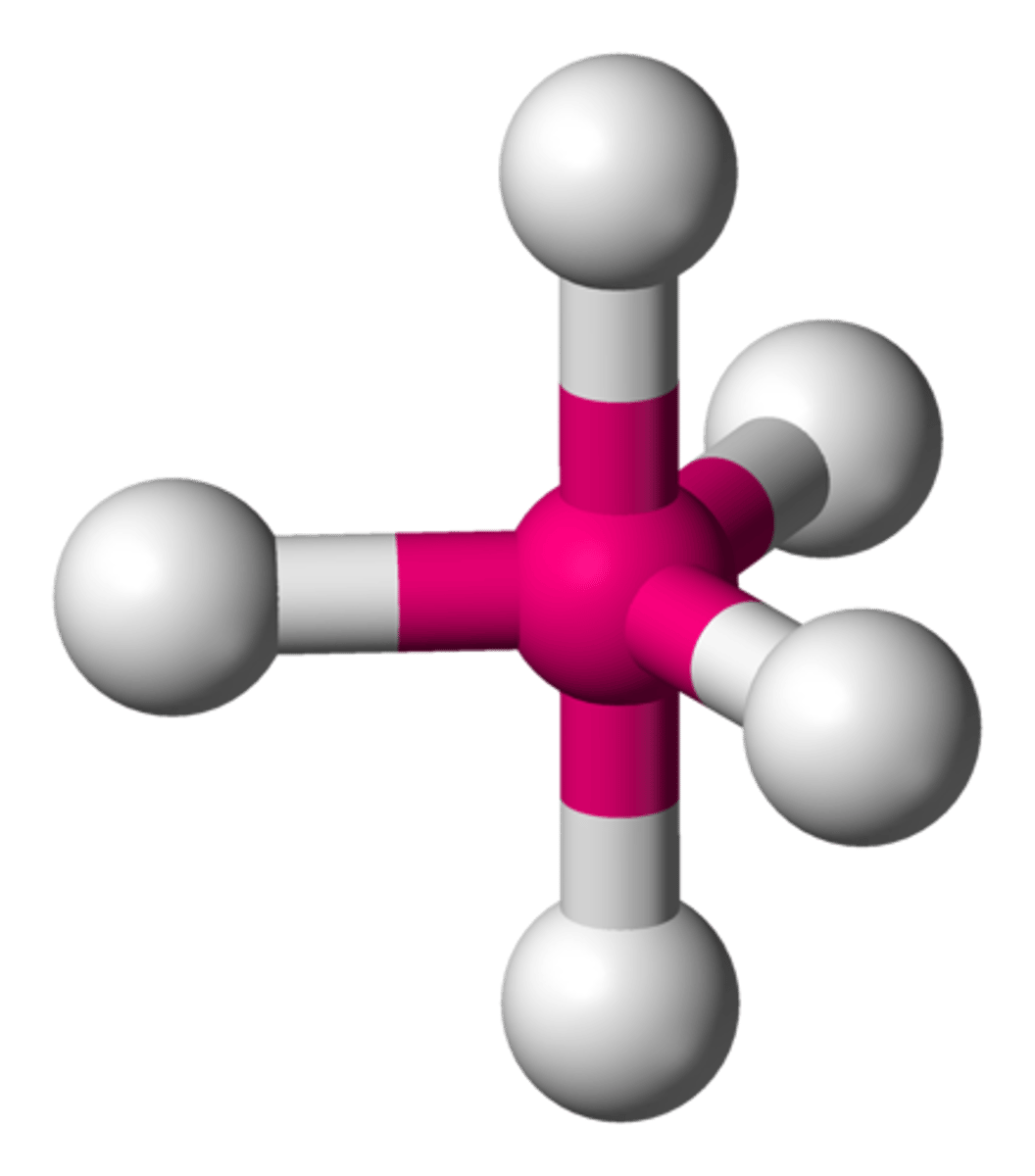
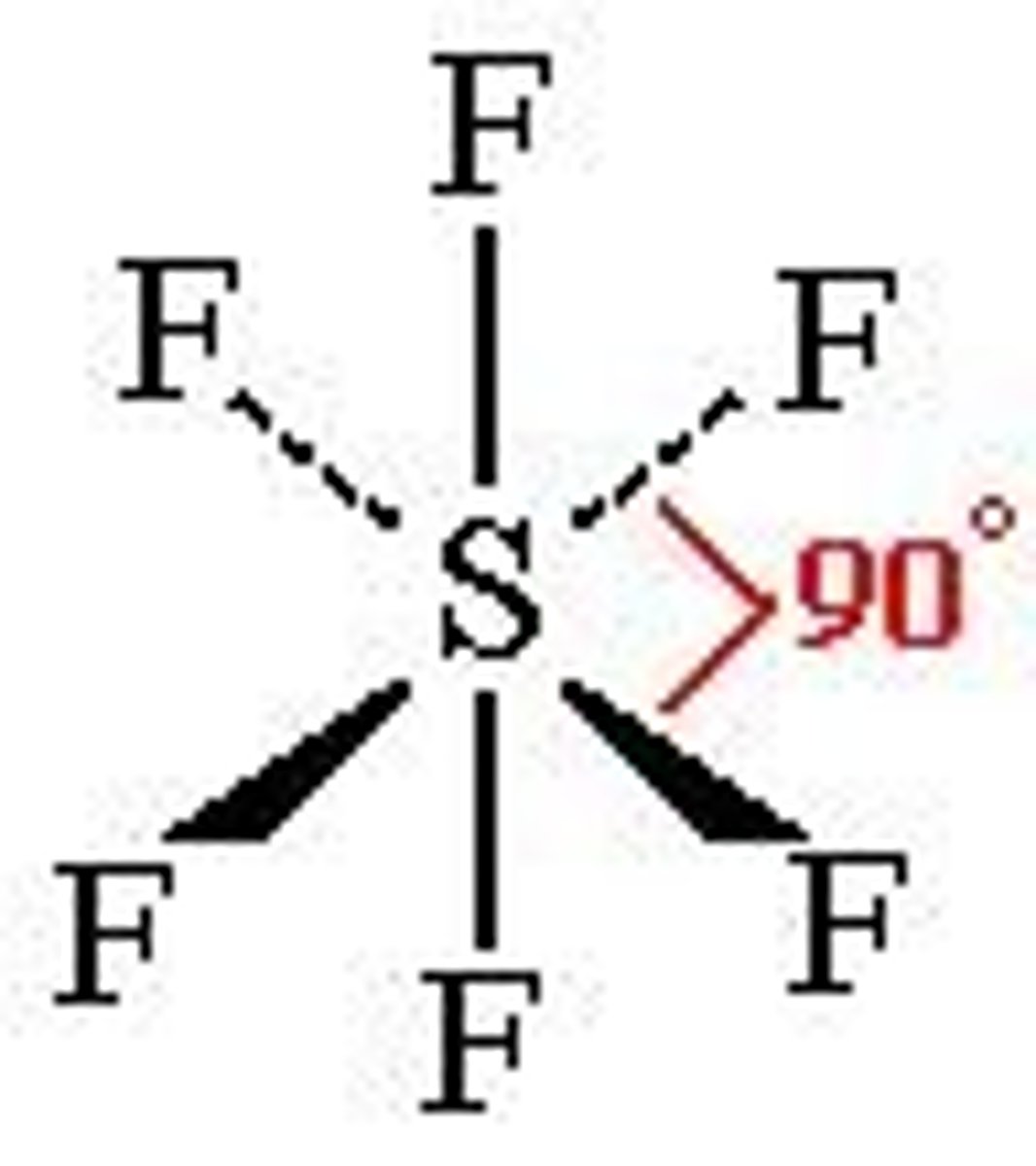
Octahedral
No. of Electron Domains 6
No. of Bonding Domains: 6
No. of Lone Pairs: 0
Square pyramidal
No. of Electron Domains 6
No. of Bonding Domains: 5
No. of Lone Pairs: 1
Square planar
No. of Electron Domains 6
No. of Bonding Domains: 4
No. of Lone Pairs: 2
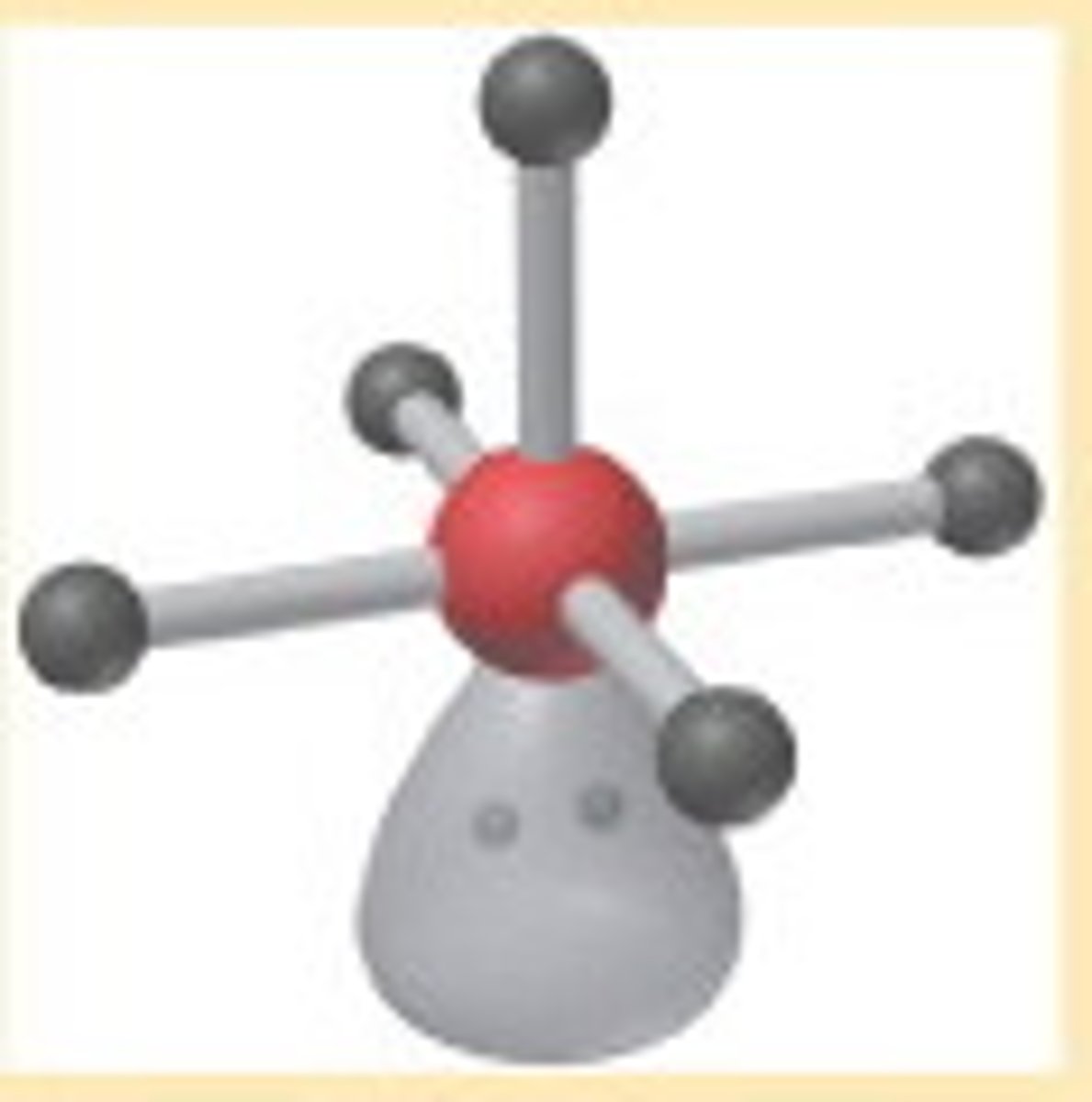
Liquids
__________ do not have a simple or regular structure, but many of their properties can be explained qualitatively by viewing them at a particular level.
Properties of Liquids
Include surface tension, capillarity action, heat of vaporization, boiling point, viscosity, and vapor pressure.
Surface Tension
The elastic force on the surface of a liquid; it's the energy required to increase the surface area by a unit. It manifests as a skin-like surface layer.

Causes of Surface Tension
Caused by intermolecular forces pulling surface molecules inward and sideways, but not outward; stronger intermolecular forces result in higher surface tension.
Surface Tension Example
Explains why small objects like needles or paper clips can float on water if placed gently and why water droplets are spherical.

Capillarity (Capillary Action)
The tendency of a liquid to rise in narrow tubes or small spaces due to attraction between the liquid and solid surfaces
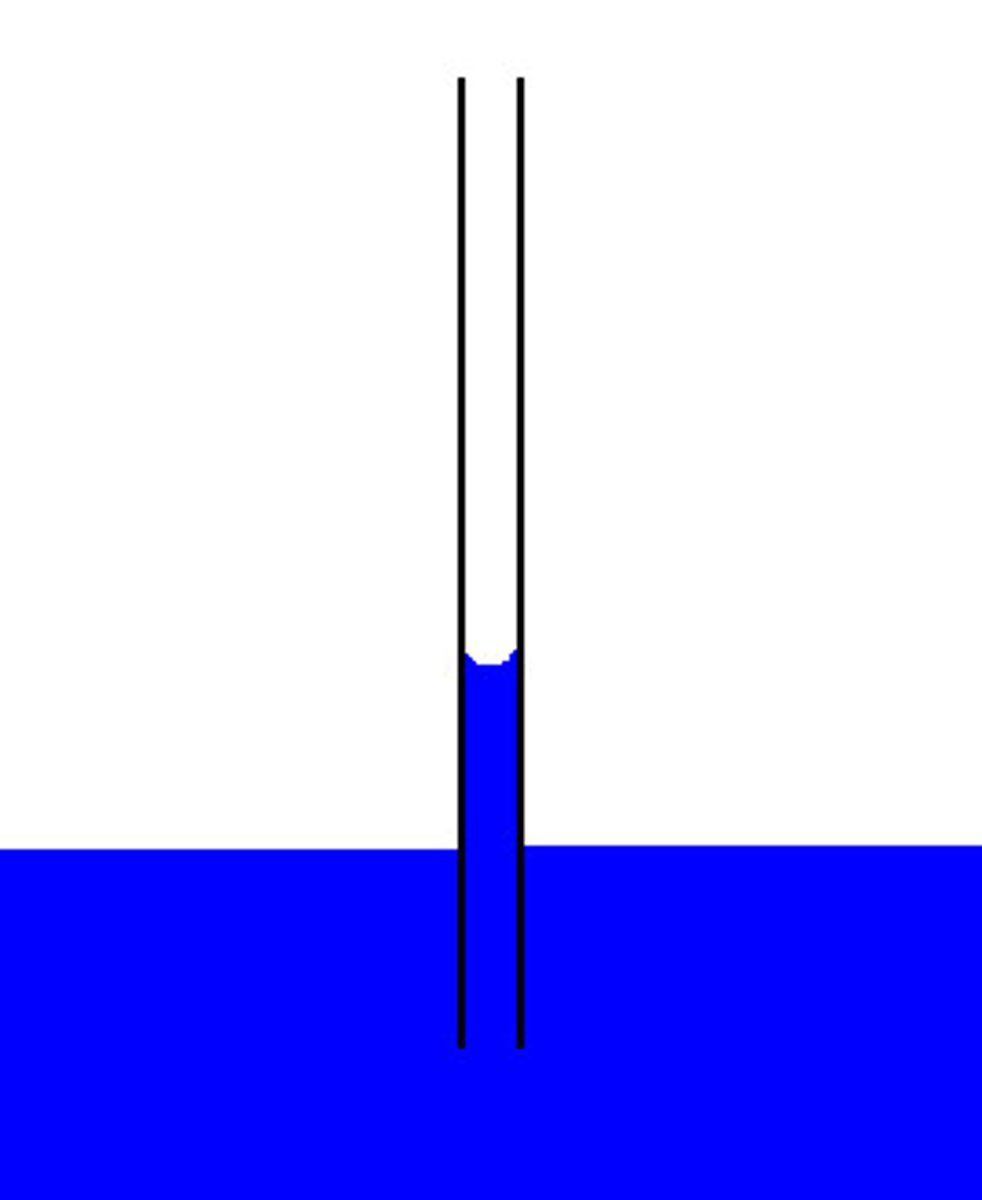
Cause of Capillarity
Results from intermolecular attraction between the liquid molecules and the solid material (adhesion), such as water molecules climbing up glass tubes.
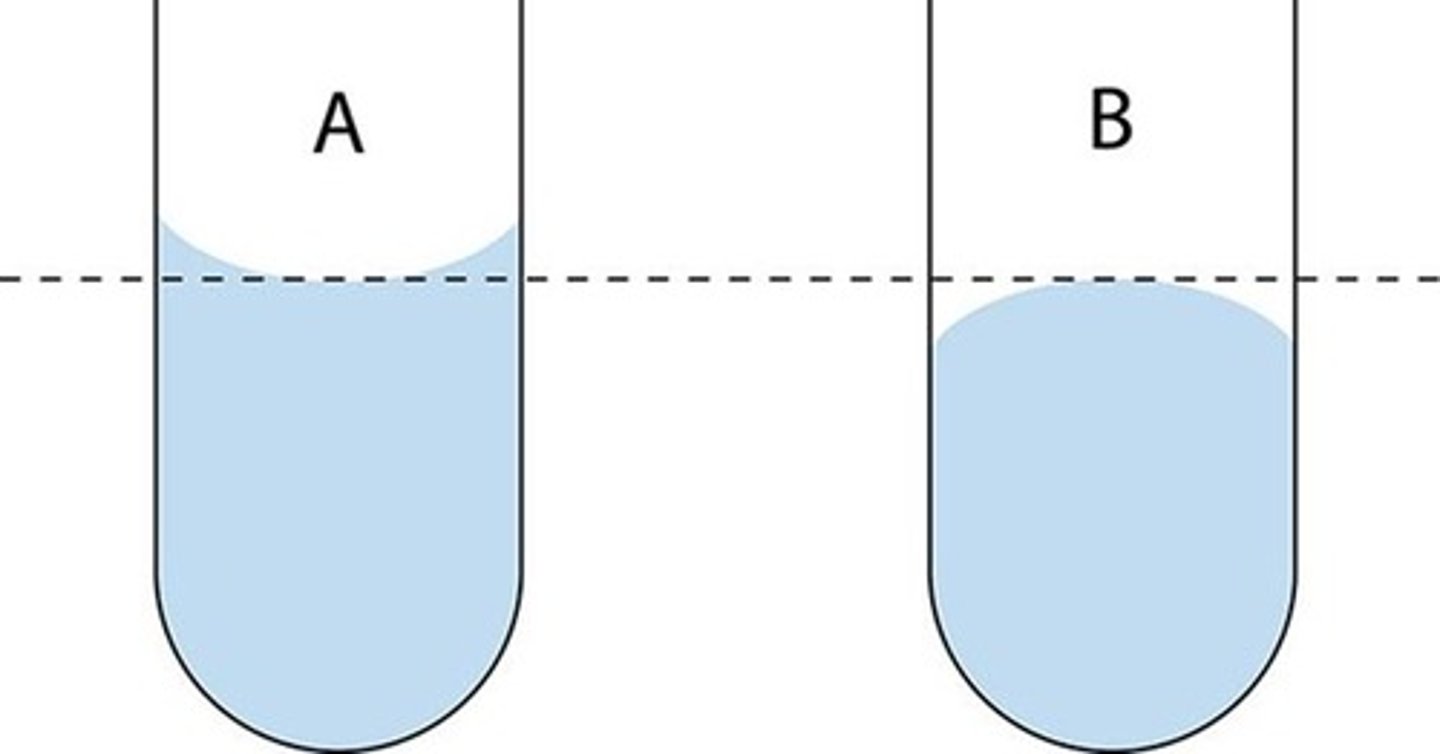
Cohesion
Attraction between like molecules (e.g., water molecules to other water molecules).

Adhesion
Attraction between unlike molecules (e.g., water molecules to glass).

Convex Meniscus
Forms when cohesive forces are stronger than adhesive forces (liquid curves upward at the surface).
Viscosity
The resistance of a liquid to flow; depends on the strength of intermolecular attractions. Stronger forces = higher viscosity.

Example of Viscous Liquids
Oil (due to long chains) and honey (due to hydrogen bonding between -OH groups) have high viscosity.

Water Pressure
The pressure exerted by a vapor in equilibrium with its liquid or solid phase in a closed container.
Example of Vapor Pressure
Water (0.03 atm) vs. Ethyl Ether (0.68 atm); stronger intermolecular forces result in lower vapor pressure.
Boiling Point
The temperature at which the vapor pressure of a liquid equals the atmospheric pressure; influenced by intermolecular forces.

Boiling Point and Altitude
At higher altitudes, atmospheric pressure is lower, so the boiling point of liquids decreases.
Heat of Vaporization (∆Hvap)
The amount of heat required to vaporize one mole of a substance at its boiling point. Increases with stronger intermolecular forces.
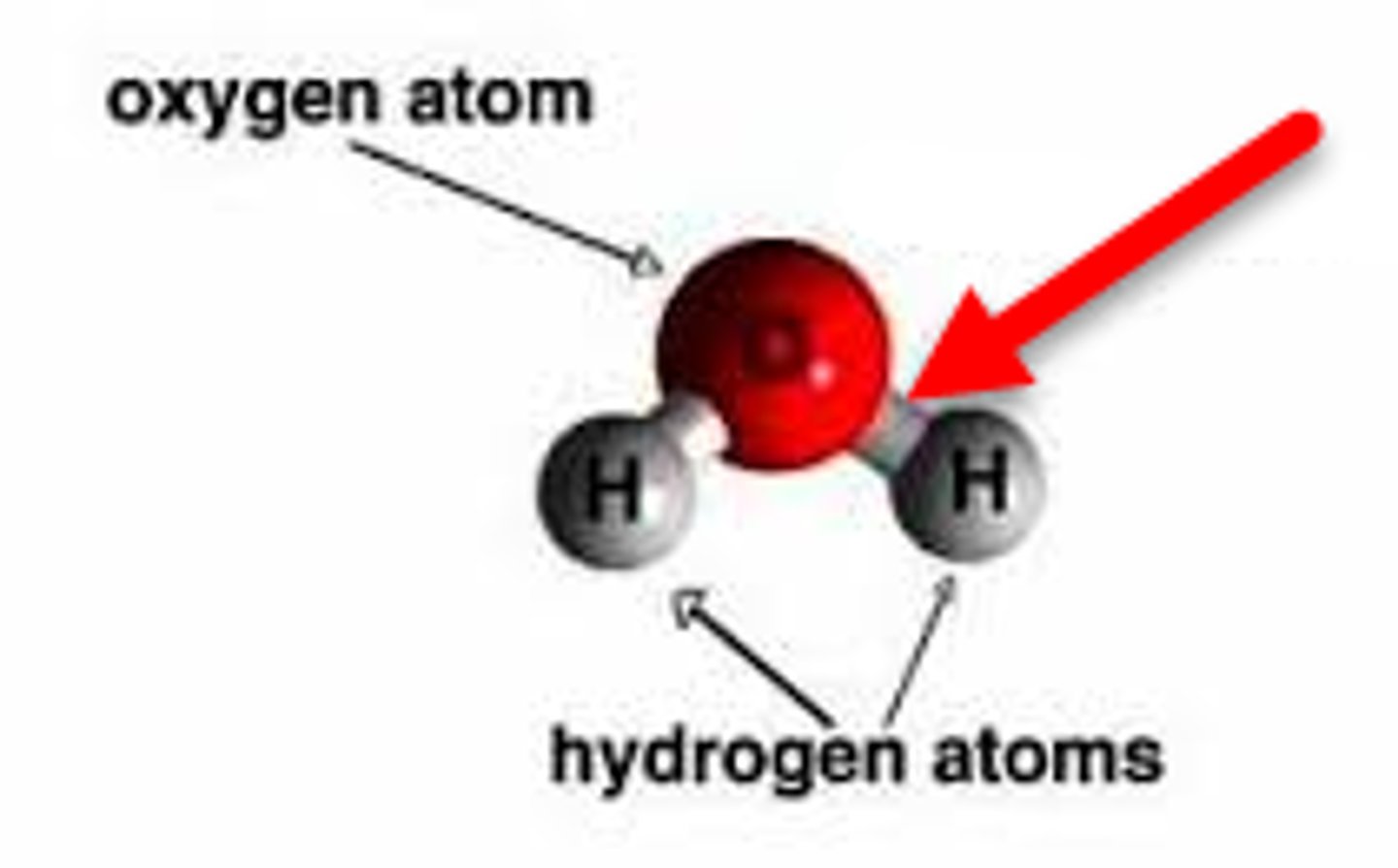
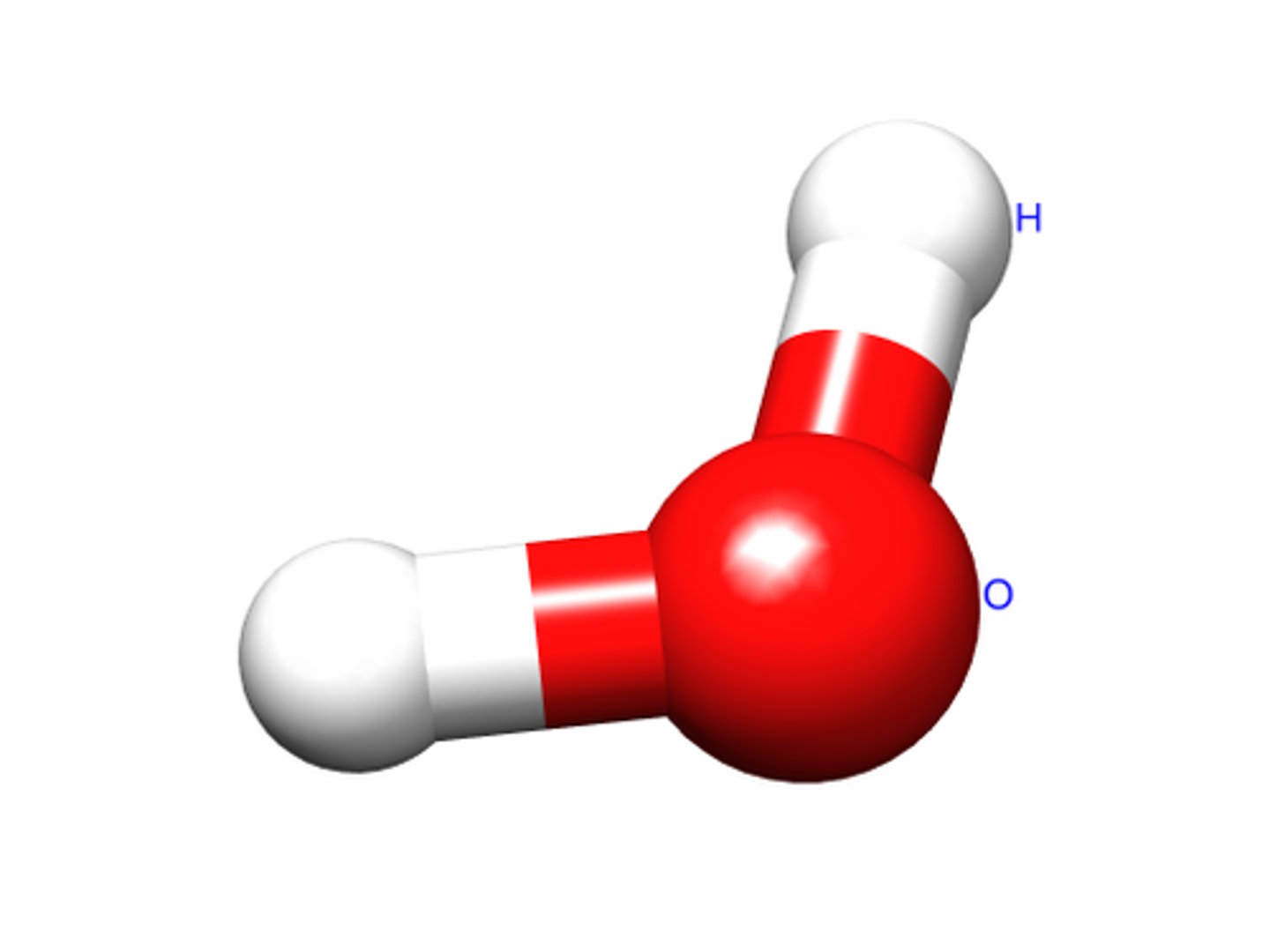
Structure of Water
At room temperature, water is colorless, odorless, and tasteless. It freezes at 0°C and boils at 100°C at 1 atm pressure.
Unique Properties of Water
- Excellent solvent
- High specific heat (energy needed to raise temperature of 1g by 1°C)
- High boiling point
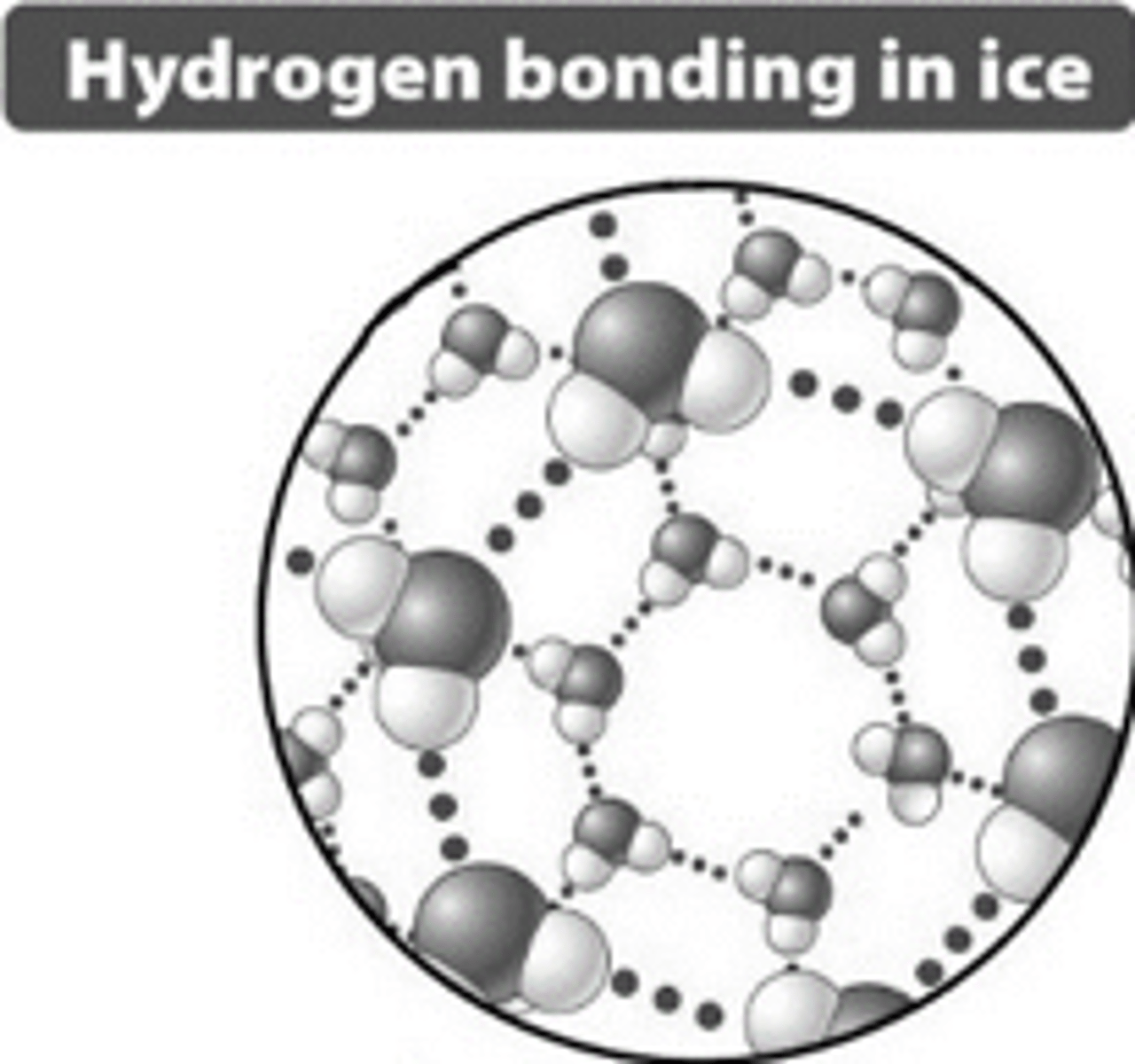
- Solid water (ice) is less dense than liquid water and floats
Ice Floats
hydrogen bonds in ice create an open structure with more space between molecules, making ice less dense than water.
A balloon expands when heated. Best explanation based on the Kinetic Molecular Theory
Gas particles move faster and exert more pressure on the balloon walls.
main idea behind the VSEPR theory
Electron pairs repel each other and determine molecular shape.
According to the KMT, it happens to the motion of gas particles when the temperature increases
The motion will also increase
explains why ionic compounds have high melting points
The strong electrostatic forces between ions require more energy to break
bond angle in a molecule with tetrahedral geometry
109.5°
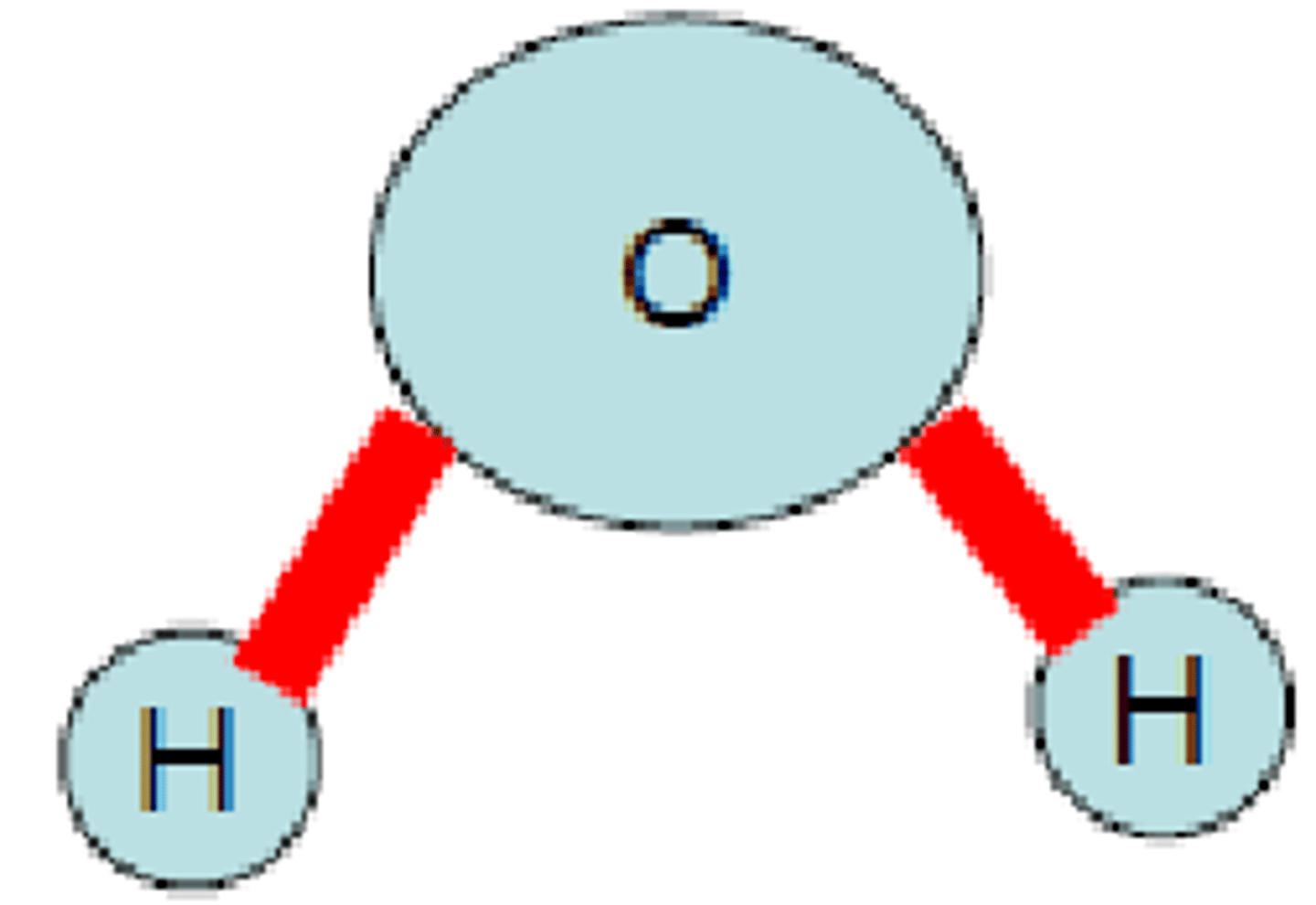
molecules has a bent shape due to lone pairs on the central atom
H₂O
causes of covalent bond to be polar
Unequal sharing of electrons due to differences in electronegativity
pair of elements is most likely to form an ionic bond
Sodium and Chlorine
Dipole-dipole interactions occur between
Two polar molecules
Hydrogen bonding can occur when hydrogen is bonded to which of the following elements
Nitrogen, Oxygen, or Fluorine
VSEPR stand for
Valence Shell Electron Pair Repulsion
only London dispersion forces
Br₂
Strongest type of intermolecular force
Hydrogen bonding
Substances with hydrogen bonding generally have higher boiling points
They are nonpolar. Hydrogen bonds are stronger than other intermolecular forces.
In VSEPR theory, the effect of lone pairs on bond angles
They decrease bond angles due to stronger repulsion.
In a molecule of water (H₂O), the type of bond holds the hydrogen and oxygen atoms together
Polar covalent bond
Intermolecular Forces of Attraction, also known as Van der Waals Forces
They are attractive forces between molecules or particles in the solid or liquid states, named after Johannes van der Waals (1837-1923).
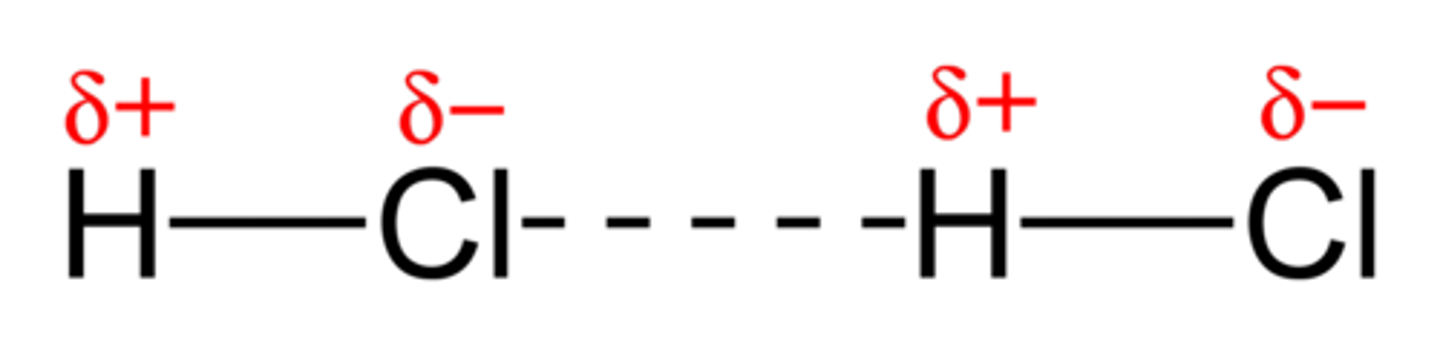
Dipole-Dipole Forces
Forces exist between polar molecules. One end of a dipole attracts the oppositely charged end of the other dipole.
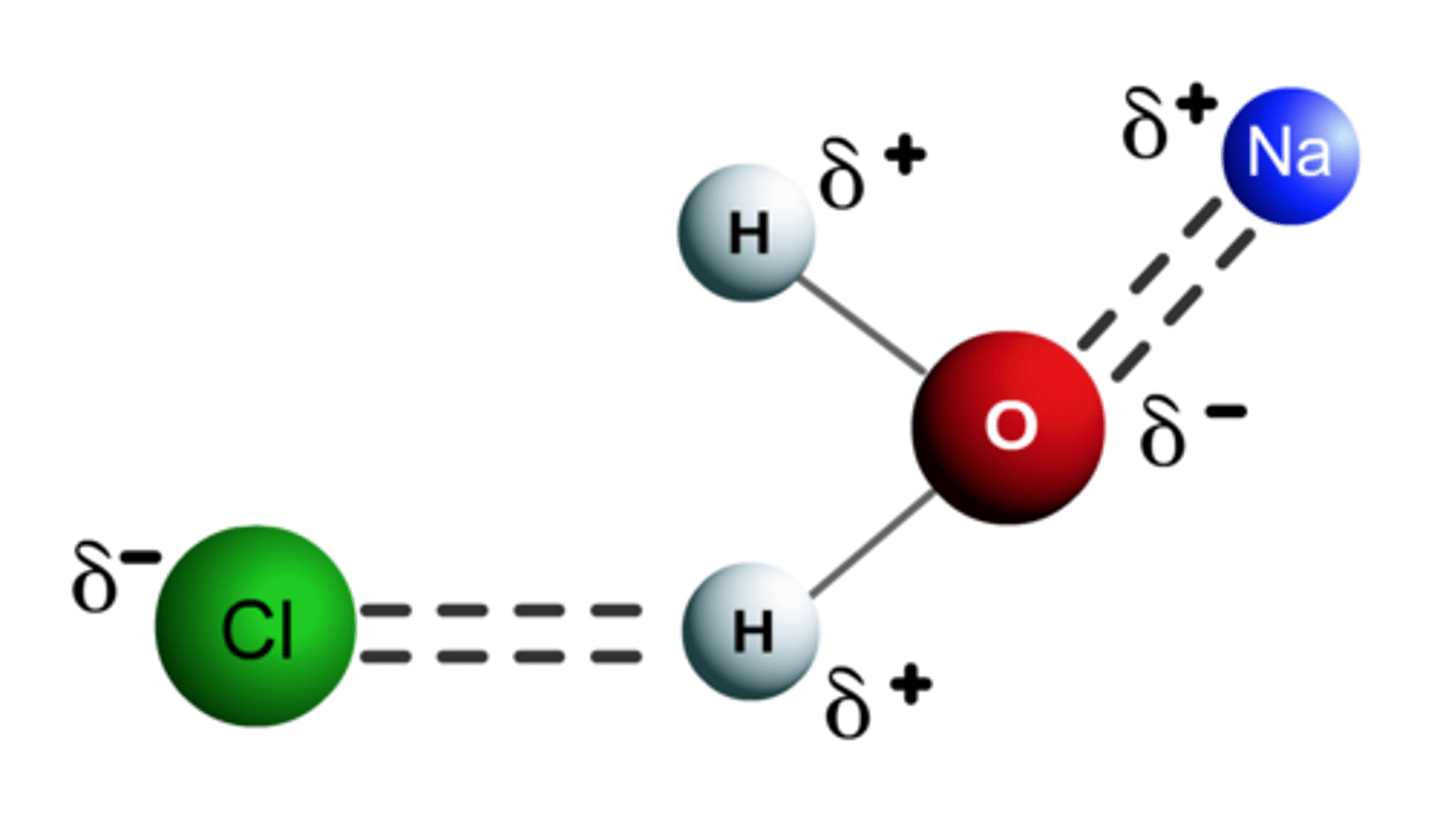
Ion-dipole Forces
occurs between an ion (either positive and negative) and a polar molecules. This explain the solubility of ionic compounds in water, which is a polar molecule. The ions and the oppositely charged ends of the polar water molecules overcome the attraction between ions themselves. Each ion becomes separated and water molecules cluster around it.
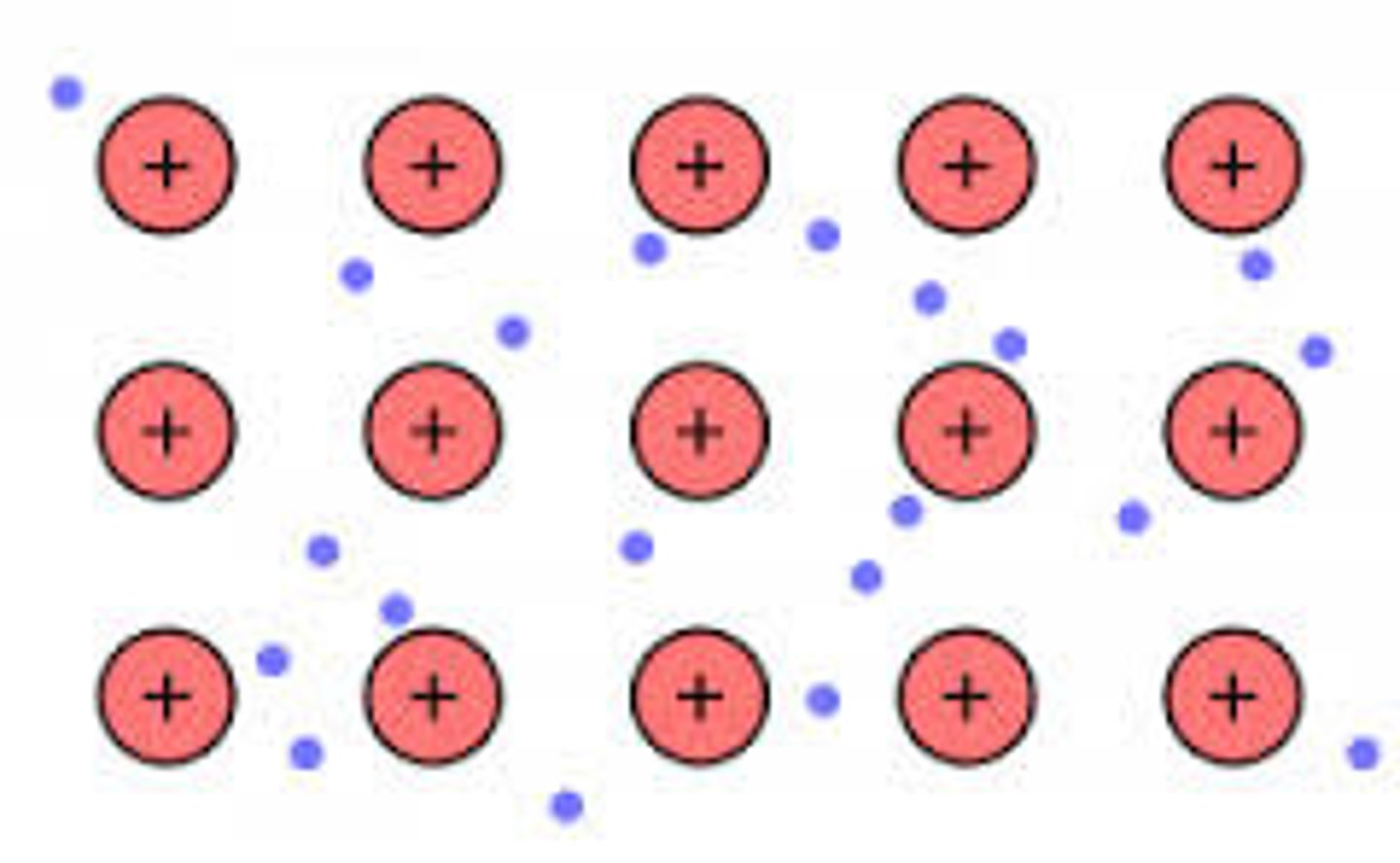
London Dispersion Forces
It is the weakest type of intermolecular force. When two non-polar molecules approach each other, an instantaneous dipole moment form. This force is sometimes called an induced dipole-induced dipole attraction
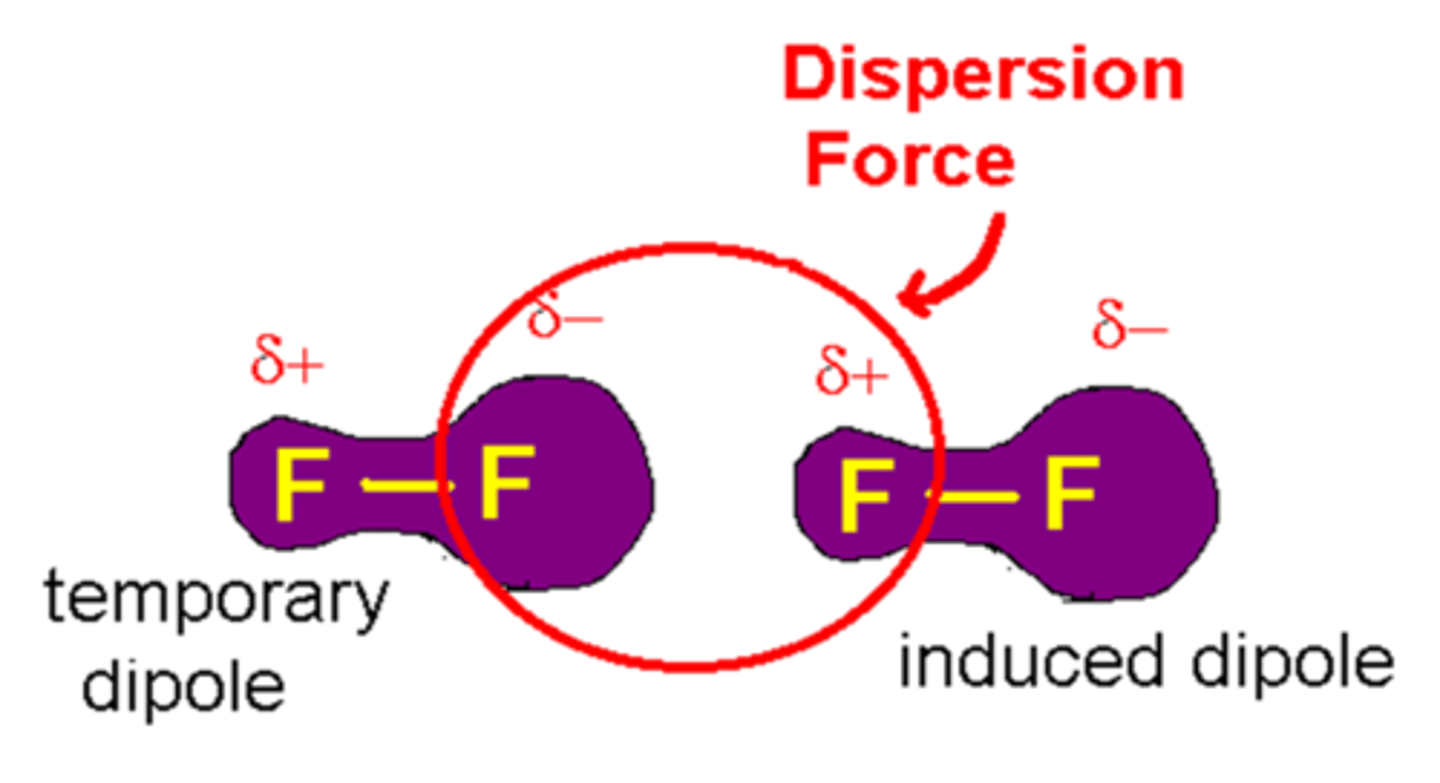
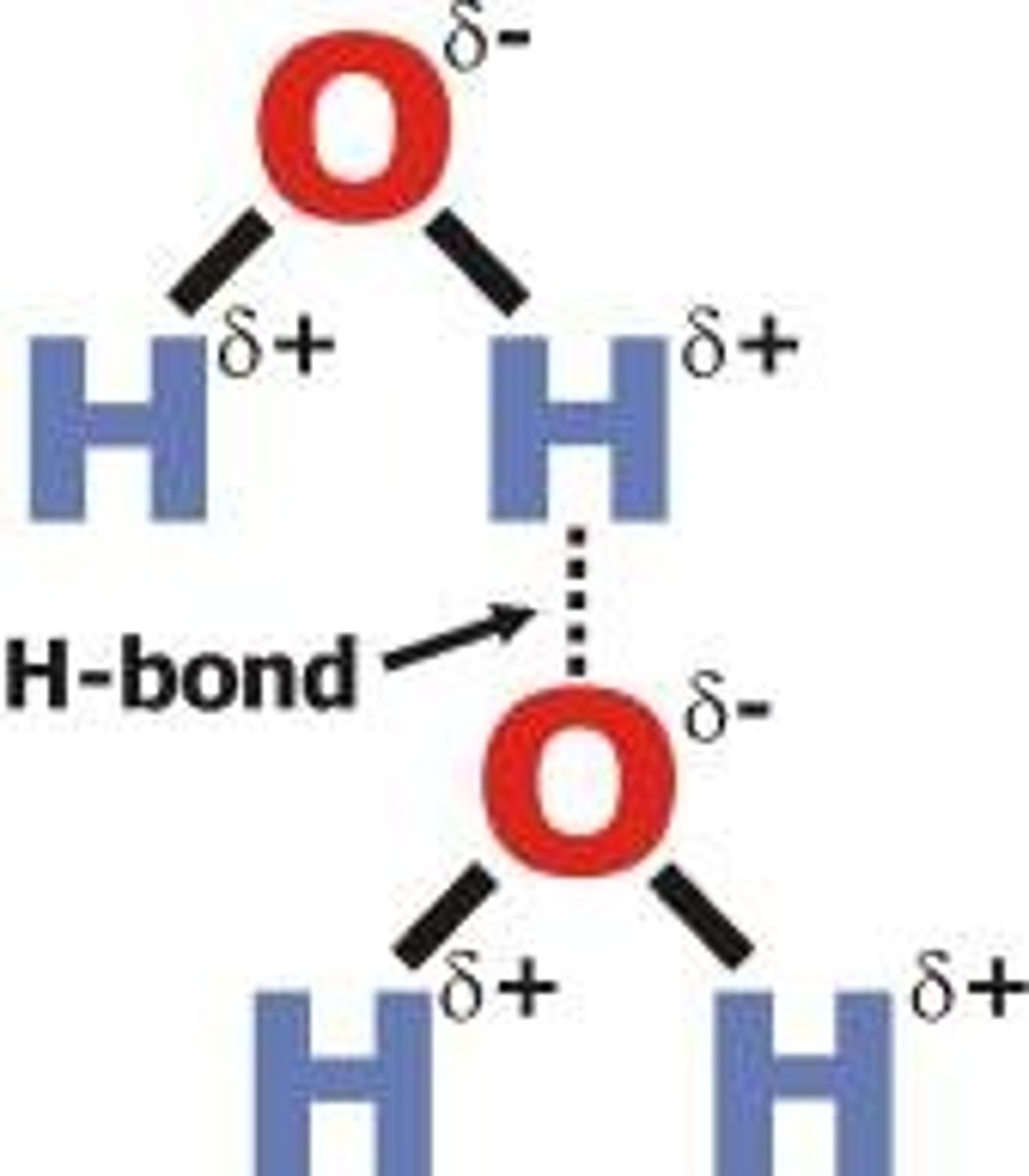
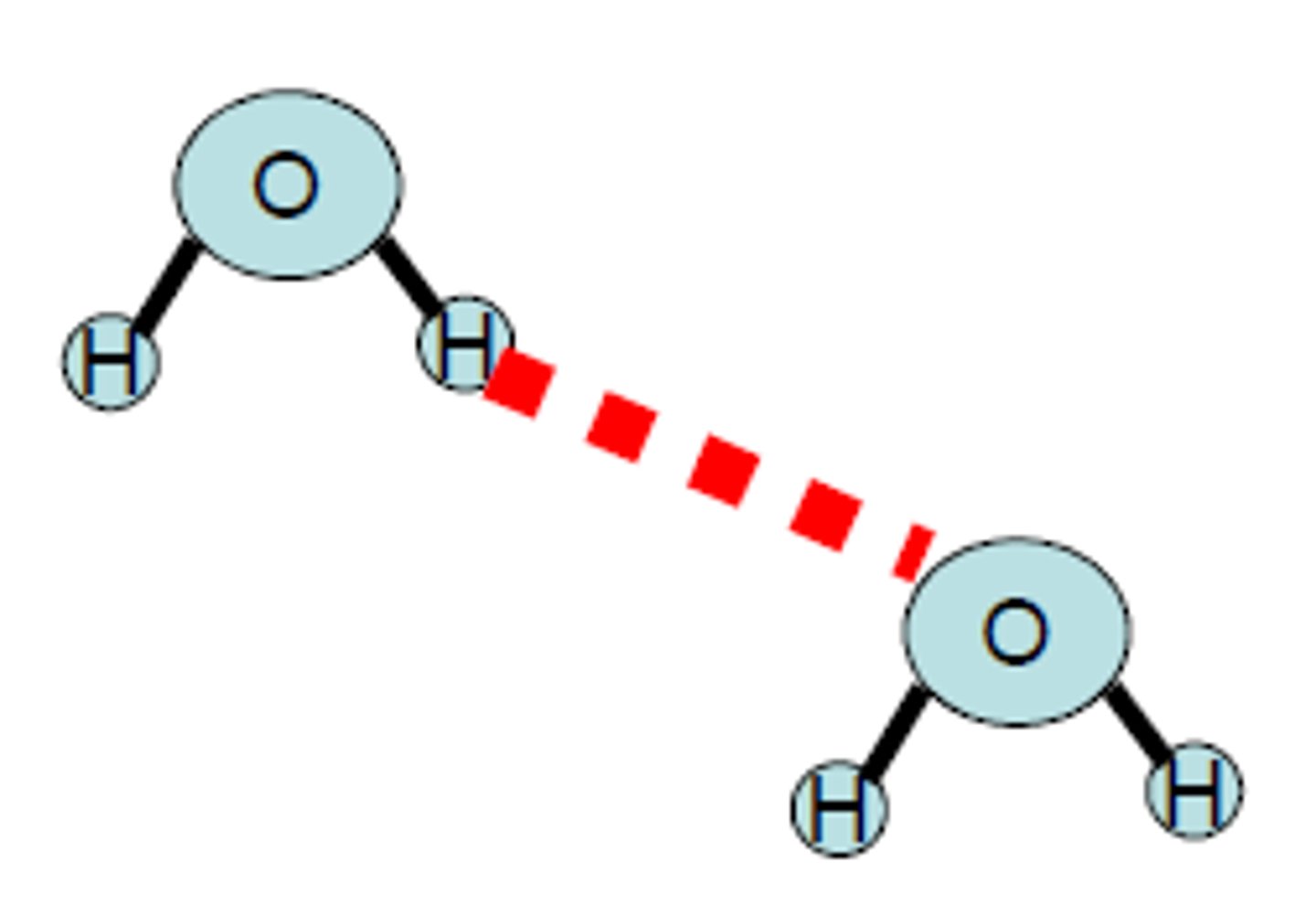
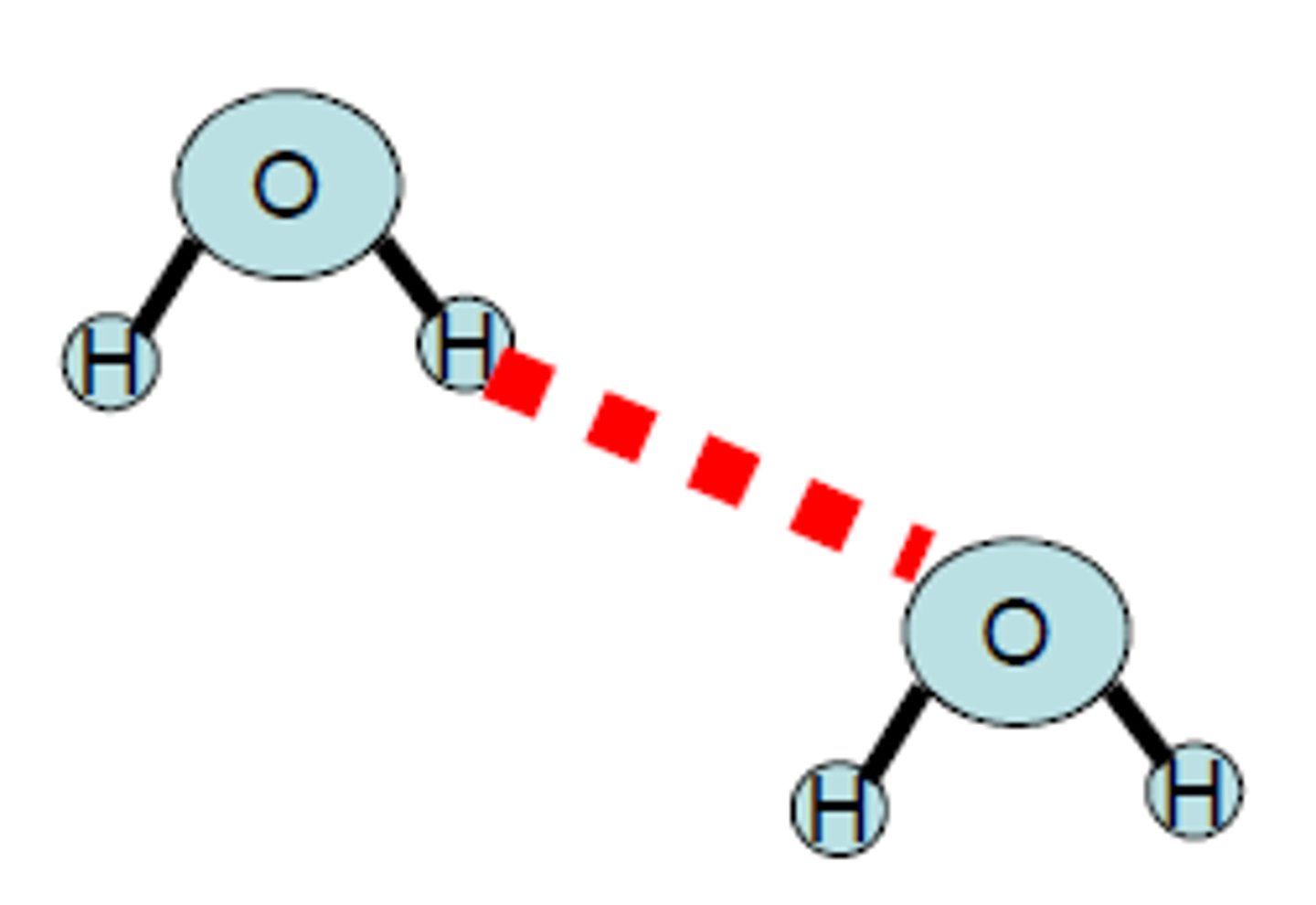
Hydrogen Bonding
it is a special and very strong type of dipole-dipole force that exists between hydrogen atom bound to a small and highly electronegative non- metal atom. A hydrogen bond occurs in polar molecules containing H and any of highly electronegative elements, in particular Nitrogen, Fluorine, and Oxygen.