IB2/U1: Thermochemistry
1/70
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
71 Terms
heat (Q)
thermal energy:
what is it
what are its properties
the form of energy that is transfered between objects or systems because of a temperature difference
energy within a system that’s created by the random motion of molecules and atoms (KE)
what is a spontaneous process?
processes that occur without the addition of energy besides activation energy (no need for continuous energy input)
what is entropy (S)
units
a measure of the degree of disorder in a system
entropy increases when there are more ways for energy to be dispersed or spread out
units: J/K mol
explain the 2nd law of thermodynamics: a reaction is spontaneous if there is an overall increase in entropy of the system and surroundings (universe) and how does this show that spontaneity is a balance between entropy and enthalpy?
its insufficient to just know the entropy change of the system OR the surroundings, if you want to figure out if a process is spontaneous. for example, the system can decrease in entropy, but if the surroundings increase enough in entropy, than there can still be a net increase. the increase in entropy in the surroundings is usually done through the release of heat from system to surroundings.
what is thermochem?
energy changes in changes of matter
Heat (Q) vs Thermal energy and temp
thermal energy: the total energy within a system because of the random motion of molecules and atoms: KE+PE (the forces between particles). this energy can be transferred, and when it is, its transferred as heat
heat: thermal energy that is transferred between objects or systems of different temp hot—> cool
temp: the average kinetic energy of the particles in a sample of matter
system vs surroundings
types of systems:
open
closed
isolated
system: the actual reaction itself, the substances undergoing the change
surroundings: the environment around the system
types of systems: affects exchange between the system and the surroundings
open: exchange energy and matter
closed: exchange energy but not matter
isolated system: cannot exchange matter or energy
enthalpy
enthalpy is the amount of heat energy contained in a substance which is stored in chemical bonds
it cannot be measured directly → we can only measure the change in enthalpy
the enthalpy of a substance is from its particles kinetic and potential energy
what is an enthalpy change
it is the amount of heat energy released or taken in per mole of substance during a physical or chemical change
examples of enthalpy change:
enthalpy change of formation ∆Hf
enthalpy change of combustion ∆Hc
bond enthalpy
the change in energy when 1 mole of a compound is formed from its elements in their standard states
the change in energy when 1 mole of a substance is burnt completely in O2
bond enthalpy is the change in energy when one mole of gaseous covalent bonds is broken (energy change of bond breaking/reforming)
bond reform = -bond break (bond break >0 always)
what is Hess’s Law
the enthalpy change during a chemical change is independant of the intermediate steps taken (state function)
as long as you have the same reactants and end at the same products, the enthalpy change is the same
RP = RI + RP
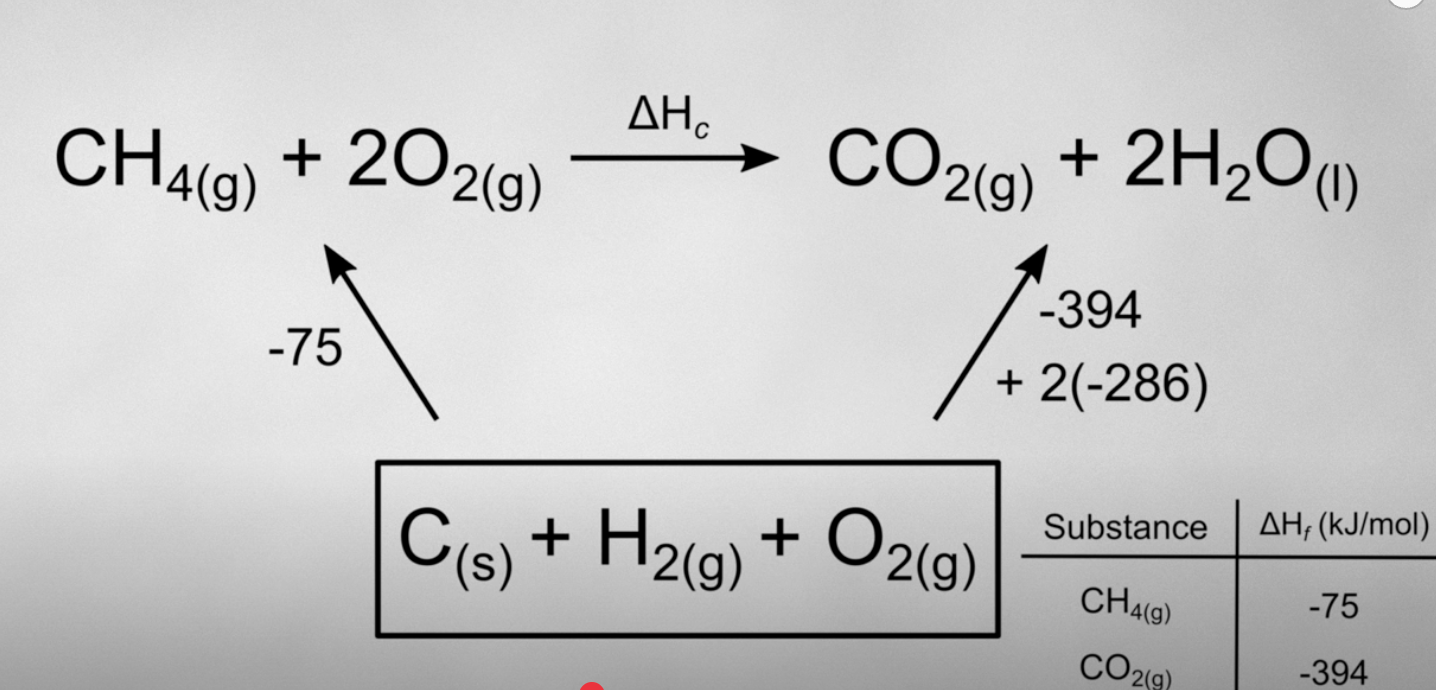
using Hess’s law, how can you find the enthalpy of combustion ∆Hc without changing the directions of the arrows?
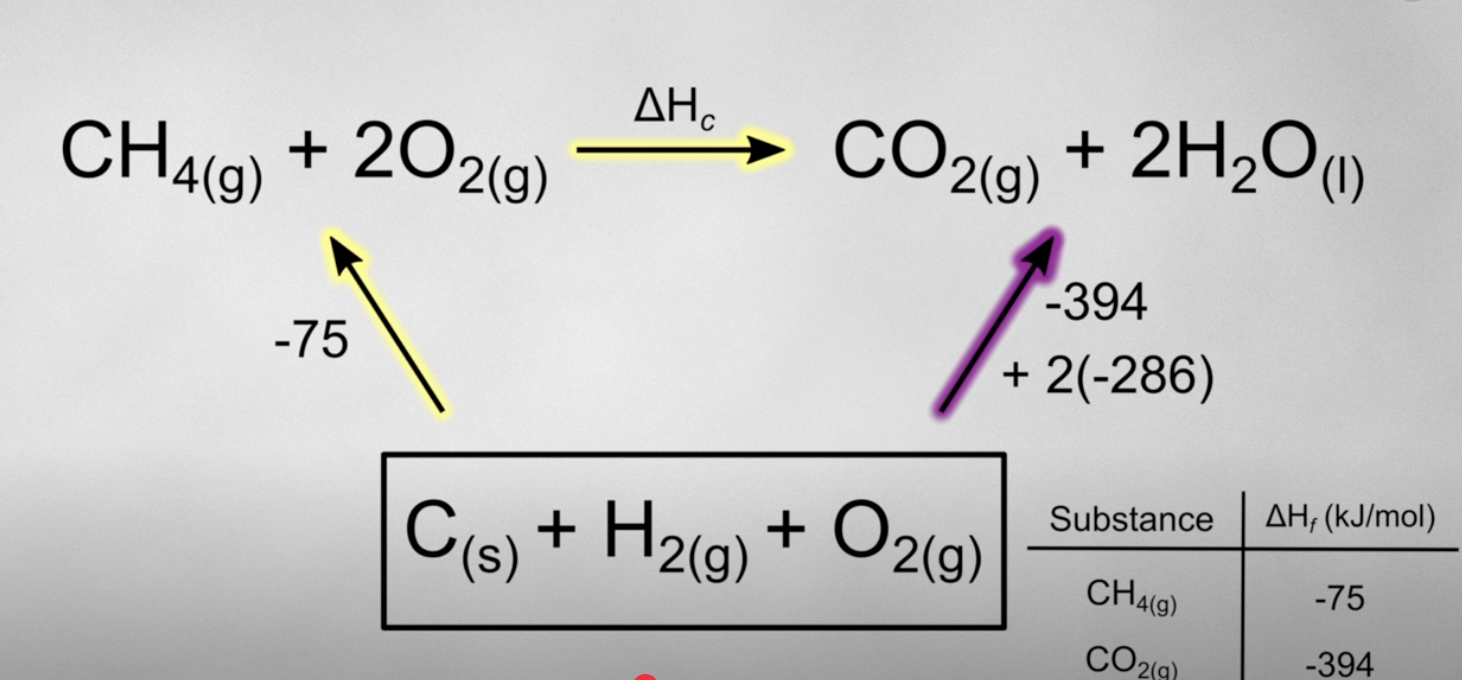
recall that Hess’s law states that intermediate steps dont matter as long as you have the same reactants and products so instead of switching the signs, just switch the path way, starting with the elements of formation as the reactants.
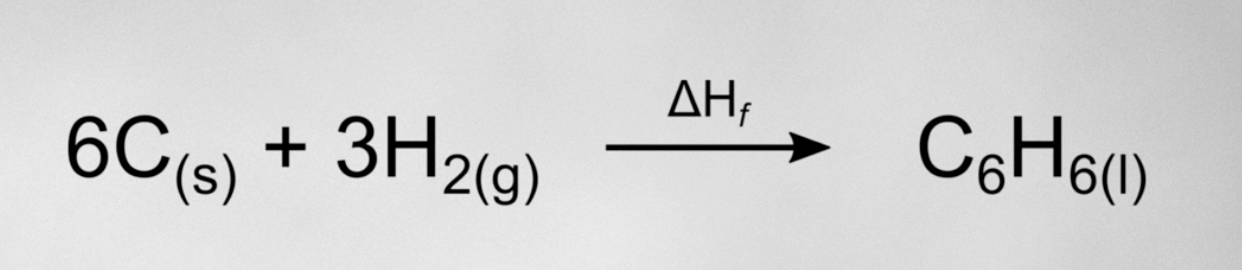
how would you solve for the enthalpy of formation if you were only given the enthalpy of combustion for C, H2 and C6H6?
*we always set up the hess cycle using whatever enthalpies we’ve been given as our intermediate step
enthalpy of vaporization
why is this important for bond energies?
the energy change when one mole of a liquid is boiled to form a gas
this is important because if we are given a substance in liquid form, we not only need to find the bond energies for the substance but also add the enthalpy of vaporization to account for the energy needed to overcome IMFs to get to a gas state
potential energy of particles
the potential energy of particles comes from the intermolecular forces between the partciles because of their reltive position to each other
kinetic energy of particles
partciles have KE because of their movement
Enthalpy: unit and symbol
It is measured in KJ/mol
The symbol for enthalpy is ΔH
what conditions are needed to measure the enthalpy change?
compounds need to be in their standard states and under standard conditions: 298K, 101KPA, 1 mol/dm3 solution
system vs surrounding
the system is the reaction mixture while the surrounding is everything around it
standard enthalpy change of a reaction
enthalpy change when molar quantities of reactants in their standard states react to form products in their standard states states under standard conditions
calorimetry
calorimeter
the process of measuring energy changes during a phyiscal or chemical process
calorimeter: is an insulated reaction vessel where the change in temperature of the surroundings can be measured
specific heat capacity ( c)
specific heat capacity of water
it is the amount of heat needed to raise 1g of a substance by 1 K
water has a very high specific heat capacity! it needs a lot of energy to change its temperature! c = 4.184 J/gK
calorimetry assumptions + limitations
we assume no heat is being transferred between the calorimeter and the outside environment (making it an isolated system) but the system is rarely isolated, it may be closed though
we assume that the heat absorbed or released by the calorimeter is negligible → not true because sometimes we account for the heat absorbed by the calorimeter in calculations
we assume a dilute aqueous solution has a density and c equal to water (obvs not true) → d= 1g/cm3 c=4.184 J/gK
errors in calorimetry to mention
heat loss to atmosphere
heat absorbed by calorimeter
incomplete combustion: because its less efficient and releases less heat
not actually reacting at SATP (standard conditions)
enthalpy (H)
enthalpy change (∆H)
if heat is the transfer of thermal energy from warmer to colder substances, than enthalpy is the total thermal energy in a subtance. but we do not have a way of measuring this directly!
enthalpy change is the flow of thermal energy into or out of the system per mol
equations of enthalpy change
∆H = H(products) - H(reactants)
∆H = -Q/n
∆H = n∆Hx
molar enthalpy ∆Hx
x = subscript for the type of change (ie. comb, vap, neut)
this is the enthalpy change for 1 mol of a substance so the units are J/mol
this is different from the standard enthalpy of combustion. to calculate this, you will be calculating an enthalpy change for some amount of substance other than 1 mol using:
∆H = n∆Hx → you can use mol ratios to manipulate the molar enthalpy to help get other values
enthalpy change of neutralization ∆Hn
this is the energy change when 1 mol of water is formed from the reaction of an acid with a base under standard conditions
so the units are: KJ/mol H2O
thermochem is the study of energy changes due to changes in matter. matter changes can be ___(3)?
changes in matter can be:
physical: substance changes in appearance not identity (same formulas)
chemical: new substance produced (new formulas) with new properties - same elements rearranged
nuclear: formation of completly new elements because there is a change to the nucleus of atoms
bond dissociation energy (bond enthalpy)
the energy required to break one mole of a chemical bond in a specific compound in gaseous state
is bond enthalpy positive or negative
bond enthalpy is always positive because bond breaking requires energy
average bond energy
average bond energy is the enthalpy change when 1 mol of a chemical bond in gaseous state are broken and averged over multiple similar compounds
how is bond energy affected by the bond order: single vs double
bond order?
bond order: the amount of bonds per atom = #bonds total/ # bond domains
as bond order increases, the atoms are held closer together because more electrons are shared between them: high bond order = shorter bond length
more shared electrons = stronger bonds and stronger attraction between atoms = more energy needed to break bond
what is the ozone layer
this is a region of earths stratosphere that absorbs most of the Sun’s harmful UV radiation
its made of high concentrations of ozone (O3)
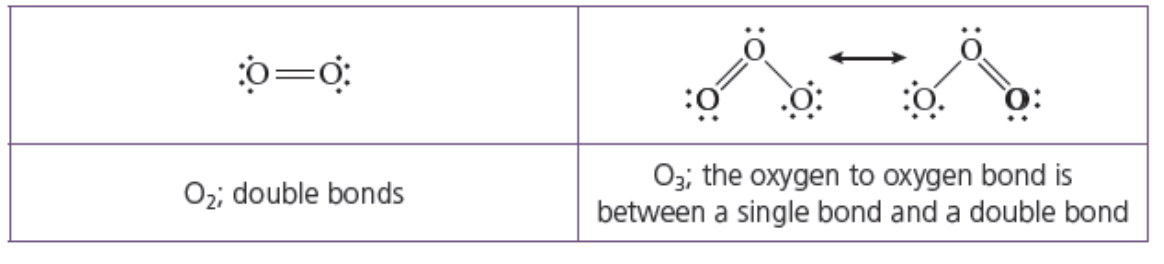
Which molecule has a higher bond order? Why?
Which bond should require more energy to break (greater bond enthalpy)?
Which bond is more stable?
O2 has a higher bond order 2 vs 1.5
because O2 has a higher bond order, it has more electrons being shared between atoms = stronger attraction of atoms to the localized electrons = stronger bond and it will take more energy to break the bond
higher bond order= stronger bond= lower PE = more stable
explain the chapman cycle
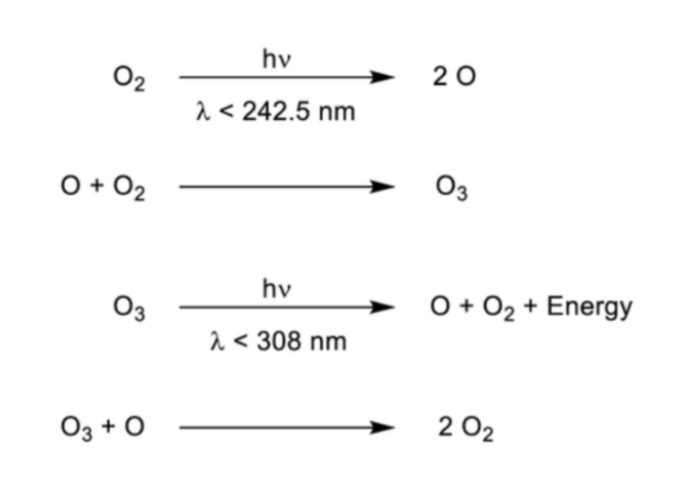
*note: short wavelength = high energy
because O2 has a higher bond order, it takes more energy to break (its broken by higher energy UV rays) → homolytic fission of O2 forms O radicals
O radicals react with O2 to MAKE O3
O3 has a lower bond order so it can be broken by lower energy UV rays → this makes an O radical and O2
O3 reacts with O radical to make 2 O2
this cycle is the natural formation and depletion of O3 where O3 is formed then used up to mkae O2
explain CFCs and Ozone depletion
CFCs are very stable in the lower atmosphere because their C-Cl and C-F bonds are pretty stable
when CFCs reach the stratosphere, theyre exposed to high energy UV radiation which creates chlorine free radicals
the C-Cl bond breaks because the C-F bond is stronger because F is more EN and it is a smaller atom which increases the strength of the C-F bond
once formed, Cl radicals start reacting with O3 to form O2 and more Cl radicals:
1⃣ Cl• + O₃ → ClO• + O₂
(chlorine radical reacts with ozone, destroying one ozone molecule)
2⃣ ClO• + O• → Cl• + O₂
(the radical is regenerated and can destroy more ozone)
This is a catalytic cycle — the chlorine radical isn’t used up.
A single Cl• can destroy thousands of ozone molecules = depletion of O3
WHY doesnt Cl radicals react with O2 then? to remake O3?
The chlorine radical has enough energy to break the weaker O–O bonds in ozone (1.5 order) but not the stronger double bond in O₂.
explain the crystal lattice around ionic compounds
in ionic compounds, ions are held in a lattice structure of alternating ions:
each ion is completely surrounded by ions of the opposite charge
the attraction between ions is called the electrostatic attraction which are very strong and have very high melting points
very brittle
explain lattice enthalpy ∆Hlat
so bond enthalpy is the amount of energy needed to break 1 mole the bonds of covalent bonds in gaseous molecules
LATTICE ENTHALPY:
Enthalpy change when one mole of a solid ionic compound is converted to its gaseous ions under standard conditions
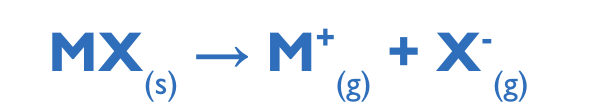
lattice enthalpy refers to ionic compounds not the individual bonds (C-H bonds)
it represents the strength of electrostatic attraction between -ions and +ions
bond enthalpy (covalent!) general equation
XY(g)→X(g)+Y(g)
*watch the states!
ex. H–Cl(g)→H(g)+Cl(g) ΔH=+431 kJ/mol
compare bond enthalpy vs lattice enthalpy:
type of substance
enthalpy reaction
endo vs exo?
depends on what 2 main factors?
Property | Bond Enthalpy | Lattice Enthalpy |
|---|---|---|
Type of substance | Covalent molecules | Ionic compounds |
Process described | Breaking 1 mol of covalent bonds | Formation or breaking of ionic lattice |
Energy change | Endothermic (breaking bonds) | Endothermic |
Units | kJ/mol | kJ/mol |
Depends on | Bond order, polarity | Ionic charge, ionic size |
Example | H–Cl bond: +431 kJ/mol | NaCl lattice: –787 kJ/mol |
🔥 Summary:
Bond enthalpy → strength of a specific bond in a molecule.
Lattice enthalpy → strength of ionic attractions in a solid crystal.
what are the 2 factors determining lattice enthalpy
ionic charge:
higher charges = stronger electrostatic attraction
this leads to increased lattice enthalpy
ion size
smaller ions have a stronger force of attraction because the distance between their nuclei to the shared electrons is less
can we directly measure lattice enthalpies?
No. lattice enthalpies cannot be measured directly
so instead we use born-haber cycles which are special energy cycles used to indirectly calculate lattice enthalpies
what is the enthalpy of atomization?
when 1 mol of gaseous atoms are formed from an element in its standard state:
Na (s) → Na (g)
define ionization energy
define electron affinity
the energy needed to remove 1 mole of electrons from 1 mole of gaseous atoms
Na (g) → Na+ (g) + e-
the energy released when 1 mol of electrons are added to 1 mol of gaseous atoms
Cl (g) + e- → Cl- (g)
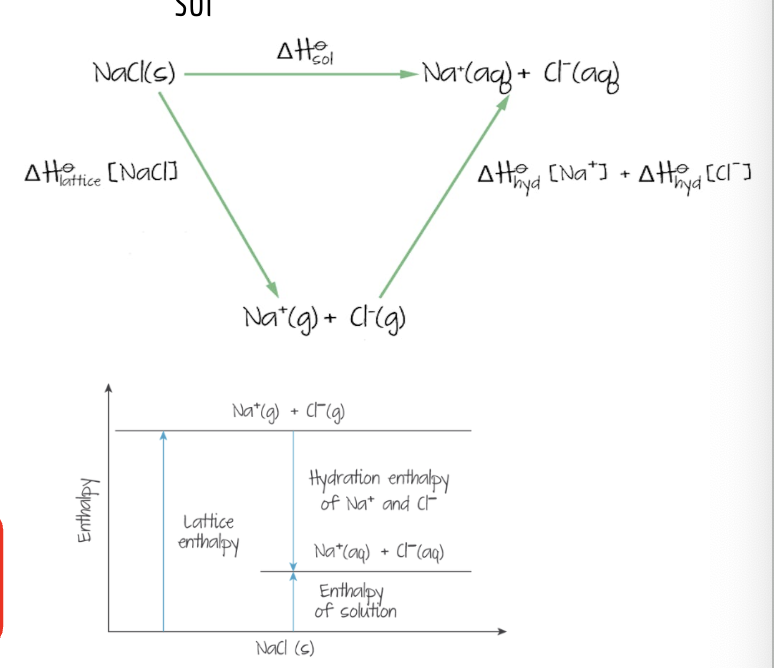
enthalpy change of solution ∆Hsol
enthalpy change when 1 mol of solute dissolves to form a solution of infinite dilution (aka a solution with a lot of excess water)
in an enthalpy change of solution, the ions in an ionic compounds dissociate to get ions with an aqeous state. the intermediate step is getting the ions in gas state → this is lattice enthalpy!
∆Hsol = ∆Hlat + ∆Hhyd
*enthalpy of hydration is turning the gaseous state ions into aq state
a combustion reaction involves what 2 important reactants?
fuel: which is a combustible substance
O2 gas
produces metallic or non metallic oxides
combustion of metals
when metals react with O2 to form metallic oxides, ex. the formation of MgO. this combustion reaction can produce lots of heat and light making it exothermic
what is rusting?
when less reactive metals combine with O2 to form metallic oxides ex. CuO. so rusting is a type of combustion reaction
can combustion take place without O2?
no
combustion of non metals
when non metals react with oxygen to form non-metallic oxides
combustion of organic compounds (carbon based compounds)
organic compounds undergo complete combustion reactions that only produce CO2 and H2O
its exothermic because even though it takes energy to break the bonds between atoms in the fuel and the bonds in a molecule of O2, the products are more energetically stable than the reactants
what is incomplete combustion?
somewhat limited O2?
very limited O2?
when fuels undergo combustion in low oxygen environments they produce H2O and CO2 but other byproducts. less O2 means CO and C can be formed.
water in solid fuels can cause incomplete combustion
somewhat limited O2 → CO + H2O
very limited O2 → C + H2O
what is the reaction for the formation of CO2?
is it complete or incomplete combustion?
what about the formation of CO?
C + O2 → CO2, this is a complete combustion reaction because the products dont include C, CO
the formation of CO is incomplete: 2C + O2 → 2CO
what are the 3 main categories of fossil fuels?
goal, crude oil, natural gas
coal: solid
crude oil: liquid
natural gas: gas
how can we separate the components of fossil fuel mixtures?
the components can be separated by their boiling points → fractional distillation
fossil fuels can be compared in terms of the amount of CO2 produced (this affects the greenhouse effect)
the amount of CO2 produced can be measured in the mols of CO2 per MJ of energy or
it can be measured as the volume of CO2 produced per mass (g) of fuel
energy released per unit mass → energy density by mass
units?
all fossil fuels release energy when they undergo combustion, but the amount varies depending on the fuel type. the amunt of energy can be compared per mole in thermochemical equations for a specific substance undergoing combustion.
the energy per unit mass is measrued in MJ/kg (amount of energy released per kg) or some other unit of mass
what is the specific energy of a fuel
∆Hfuel / Mmolar mass → the amount of energy produced in a complete combustion reaction per unit of mass (ex. g)
difference with energy released per unit mass:
specific energy is a theoretical property of a fuel
energy released per unit mass: is an experimental or practical value taken from combustion measurements
order of specific energy of fossil fuels from least to most
coal, crude oil, natural gas
what are the consequences of incomplete combustion (3)
less energy released per mole of fuel compared to compelte combustion
C and CO are harmful by products
unreacted fuel is fire hazard
which fossil fuel has the greatest tendency to undergo incomplete combustion?
coal has the greatest tendency to to undergo incomplete combustion because there are impurities like nitrogen, sulfur and other metals that can react with Oxygen → makes other byproducts
when these impurities consume O2, there might not be enough O2 left to react with the C in the coal leading to incomplete combustion
the larger a hydrocarbon molecule, the more easily it will undergo incomplete combustion.
the harder something is to fully oxidise (like large molecules!) increases its tendency to easily undergo incomplete combustion reactions
what fossil fuel is least likely to undergo incomplete combustion?
crude oil and natural gas mostly consist of hydrocarbons but crude oil has longer chains which require more O2 to oxidize completely so its more likely to undergo incompelte combustion than shorter hydrocarbon chains.
what is the relationship between CO2 levels and greenhouse effect
the greenhouse effect occurs when certain gases like CO2 absorb infrared radiation that has been emitted from the earths surface after being heated by the suns rays. this increases the solar energy trapped within the atmosphere leading to increased temperatures.
_____
is the fossil fuel that produces the most carbon dioxide per unit of energy and
_____
is the fossil fuel that produces the least amount per unit of energy during complete combustion.
coal, natural gas
The complete combustion of coal only produces one product, CO2, (C+O2 → CO2) and produces the least amount of energy per mole of CO2. Crude oil and natural gas produce CO2 and H2O, but longer hydrocarbon chains in crude oil have a greater C:H ratio than shorter hydrocarbon chains, therefore natural gas produces the least amount of CO2 per unit energy.
renewable vs non renewable energy sources
green vs renewable
A non-renewable energy source is consumed in a chemical or nuclear process and cannot be renewed at a rate equal to or faster than it is consumed.
green energy: reduced environmental impact
what are biofuels
how are they produced
biofuels are fuels derived from biological sources
modern biofuels are derived from plant material that is transformed via physical and chemical processes to produce usable fuels
biofuels are produced through the breakdown/decomposition of larger organic molecules into combustible short chain hydrocarbons
how do biofuels release energy?
When plants photosynthesise, the carbon in carbon dioxide is converted to glucose. Biofuels release energy when combusted from carbon that was originally absorbed by the plant as carbon dioxide. Carbon fixation is the process by which inorganic carbon (CO2) is converted into organic compounds such as glucose.
advantages and disadvantages of biofuels
advantages:
the plants used to make biofuels must absorb CO2 when performing photosynthesis which offsets the carbon emissions of the biofuel when combusted
they are more likely to undergo complete combustion because they are short chain hydrocarbons
takes less resources to refine biofuels which is less harmful to the environment (no need to mine or fractionally distill in their production)
cons:
uses lots of plant material which takes land for agriculture
combustion of biofuels still emits GHGs
how is coal and crude oil formed?
Coal, was formed from the remains of prehistoric plants that grew in vast swamps. When the plants died, they became buried under layers of mud and sand. Over millions of years, heat and pressure, combined with the anaerobic conditions, converted the decaying plant material into coal. The reduction of biological compounds that contained carbon, hydrogen, nitrogen, sulfur and oxygen led to the formation of coal, oil and natural gas. Crude oil is formed from the remains of marine organisms.