ib bio carbon quiz
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
organic chemistry
carbon based molecules make up all living things
it has a bond capacity of 4/ makes 4 covalent bonds and is a tetravalent atom
Why carbon?
carbon skeletons
chains of carbons either straight, ringed, branched, or attached to other things
hydrocarbons
non polar and hydrophobic skeletons with just Hydrogens attached
isomers
same molecular formula, but different forms
structural isomers, geometric isomers, enantiomers
What are the types of isomers?
enantiomers
mirror image isomers
functional groups
things that are attached to carbon skeleton that are involved in chemical reactions
OH
what is hydroxyl’s notation?
CO
what is carbonyl’s notation?
COOH
what is carboxyl’s notation?
NH2
what is Amino’s notation?
SH
what is sulfhydryl’s notation?
PO4-
what is phosphate’s notation?
an O bonded to C skeleton and to an H
what is hydroxyl’s form? (in words)
C double bonded to an oxygen and single bonded to a carbon skeleton
What is carbonyl’s form? (in words)
C with a double bonded oxygen, single bonded hydroxyl, and bonded to C skeleton
What is carboxyl’s form? (in words)
N bonded to C skeleton and 2 H’s
what is Amino’s form (in words)
S bonded to C skeleton and H
What is sulfhydryl’s form? (in words)
phosphate ion bonded to C skeleton (organic phosphates)
What is phosphate’s form? (in words)
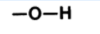
What is hydroxyl’s form? (in diagram)
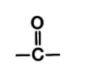
What is carbonyl’s form? (in diagram)
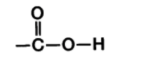
What is carboxyl’s form? (in diagram)
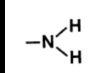
What is amino’s form? (in diagram)
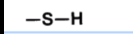
What is sulfhydryl’s form? (in diagram)