Chemistry metall
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
Metallens 4 Egenskaper
Conducts Electricity:
Metals conduct electricity due to free electrons that can move easily through the metal's structure.
Metallic Luster
: The shiny appearance of metals comes from how their free electrons reflect light.
Malleability and Ductility:
Metals can be hammered into sheets or stretched into wires because atoms can slide past each other without breaking the metal's structure.
High Melting and Boiling Points:
Strong atomic bonds in metals require a lot of energy to break, leading to high melting and boiling points.
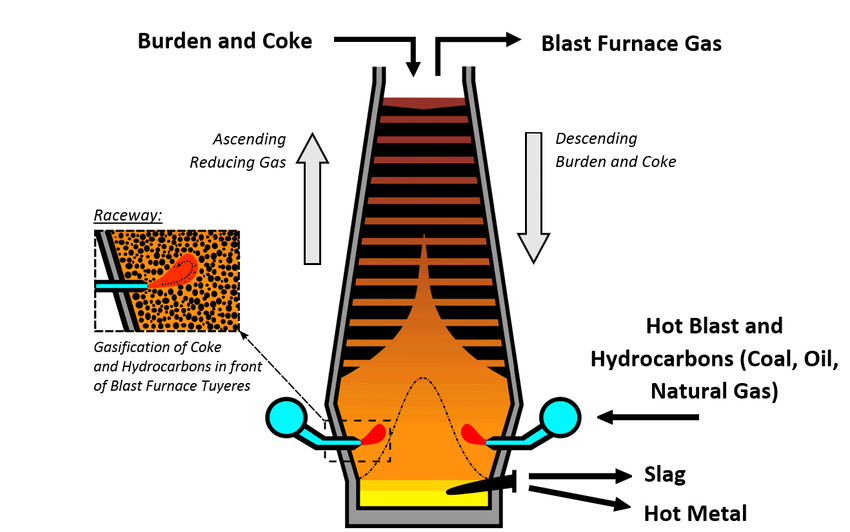
Iron Extraction Blast Furnace Process
• Blast Furnace Process: Iron is extracted from ore using coke (carbon) to reduce iron oxide into pure iron:
◦ Coke reacts with oxygen → carbon dioxide.
C + O₂ → CO₂
◦ Carbon dioxide reacts with coke → carbon monoxide.
CO₂ + C → 2CO
◦ Carbon monoxide reduces iron ore → molten iron.
Fe₂O₃ + 3CO → 2Fe + 3CO₂.
• Environmental Impact: Carbon dioxide (CO₂) emissions and energy use are the major environmental concerns.
.. Coke reacts with oxygen to create carbon dioxide: . 2. Carbon dioxide then reacts with more coke to convert into carbon monoxide: CO₂ + C → 2CO. 3. Carbon monoxide reduces iron ore into molten iron: This entire process emits carbon dioxide (CO₂) as a significant environmental concern, and the energy consumption during extraction is also noteworthy.
Miljöpåverkan vid Metallframställning (Environmental Impact of Metal Production)
CO₂ Emissions
Impact: CO₂ is a major climate change contributor, released during metal extraction, especially iron and aluminum, from the burning of fossil fuels like coal.
Recycling
Energy Savings: Recycling metals, like aluminum, saves up to 95% of the energy needed for extraction.
Less Pollution: Recycling reduces the need for mining, cutting down waste and pollution.
Resource Conservation: Recycling helps preserve resources and lowers the environmental impact of future mining.
Mining and Pollution
.
Water Pollution: Chemicals used in mining can contaminate nearby water sources, harming aquatic life.
Energy Use
Ekosystem Damage
: Mining disrupts habitats, causes soil erosion, and destroys forests
High Energy Demand:
Metal extraction requires a lot of energy, often from non-renewable sources like coal, which leads to further environmental harm.
Alloys: Mixtures of metals to improve properties
An alloy is a mixture of a metal with one or more other elements (usually other metals) to improve its properties, such as strength, hardness, or resistance to corrosion.
Steel = Iron + Carbon → Stronger, harder, used in buildings and tools.
Stainless Steel = Iron + Chromium + Nickel → Rust-resistant, used in cutlery and medical tools.
Bronze = Copper + Tin → Corrosion-resistant, used in statues and coins.
Brass = Copper + Zinc → Strong, corrosion-resistant, used in instruments and fittings.
Aluminum Alloys = Aluminum + Magnesium/Silicon → Lightweight but strong, used in airplanes and cars.
Why Use Alloys?
Stronger & Harder: Alloys are tougher than pure metals.
Corrosion-Resistant: Less likely to rust or tarnish.
More Malleable: Easier to shape without breaking.
Better Conductivity: Some alloys improve electrical or thermal properties.
Oxidation and Reduction (Redox Reaction) – Step by Step
What Happens in Oxidation?
A metal atom loses electrons, forming a positive ion.
This happens when metals react with oxygen, water, or acids.
Example: Iron Oxidizing
Iron (Fe) reacts with oxygen from the air.
It loses 2 electrons, turning into an iron ion (Fe³⁺).
Reaction:
Fe → Fe²⁺ + 2e⁻
Why Does This Happen?
Iron wants to be more stable, so it gives away electrons.
The lost electrons don’t disappear—they are taken by another substance (like oxygen).
This means oxidation and reduction always happen together (redox reaction).
What Happens Next?
The lost electrons go to oxygen, which gains them (reduction).
This forms iron oxide (rust) when iron and oxygen combine
Why It Matters
Redox reactions occur in batteries, corrosion, and metal production.
Reduction = Gaining Electrons (Oxygen Reacts)
Oxygen in the Air
The air around us contains oxygen molecules (O₂).
Oxygen doesn’t normally have a charge, but it wants to gain electrons to become stable.
Oxygen Gains Electrons
When oxygen reacts with a metal (like iron), it takes in extra electrons.
Electrons are negatively charged, so when oxygen gains them, it becomes negatively charged.
Oxygen’s Chemical Change
The oxygen molecule (O₂) gains 4 electrons to form 2 oxygen ions (O²⁻ each).
This reaction looks like this:
O₂ + 4e⁻ → 2O²⁻
What This Means
The oxygen is reduced because it gains electrons.
It now has a negative charge, which allows it to bond with positive metal ions (like Fe²⁺ from oxidized iron).
This bonding creates iron oxide (rust) when combined with water.
Why Does Oxygen Gain Electrons?
Atoms “like” having a full outer shell of electrons (it makes them stable).
Oxygen needs 2 more electrons to fill its outer shell.
By gaining electrons from metals, oxygen becomes stable and forms oxide ions (O²⁻).
Redox Reaction = Both Happen Together
What is a Redox Reaction?
A redox reaction is a combination of oxidation and reduction happening at the same time.
Oxidation: A substance loses electrons.
Reduction: A substance gains electrons.
Example: Rusting (Iron Oxidizing)
Iron (Fe) reacts with oxygen (O₂) in the air, causing rust to form.
Iron loses electrons (oxidation), and oxygen gains electrons (reduction).
This reaction creates iron oxide (rust), which is a compound of iron and oxygen.
Step-by-Step of Rusting:
Oxidation: Iron (Fe) loses 2 electrons, turning into iron ions (Fe²⁺).
Fe → Fe²⁺ + 2e⁻
Reduction: Oxygen (O₂) gains 4 electrons and forms oxygen ions (O²⁻).
O₂ + 4e⁻ → 2O²⁻
The iron ions (Fe²⁺) and oxygen ions (O²⁻) combine to form iron oxide (Fe₂O₃), which we see as rust.
Why Is This Important?
In redox reactions, one substance loses electrons (oxidation), and another substance gains those electrons (reduction).
This is always the case, whether it’s rusting, burning, or other chemical processes
Noble and Base Metals
Noble Metals: Do not easily oxidize. E.g., Gold, Platinum.
Base Metals: React easily with oxygen. E.g., Iron, Zinc.
Noble metals steal electrons from base metals if they are in the same place in an electrolyte solution.
This happens because noble metals are less reactive and have a stronger tendency to attract electrons than base metals.
Ädla metaller:
Guld
Platin
Silver
Koppar
Oädla metaller:
Väte
Järn
Zink
Magnesium
Kalium
Naturmaterial:
Väte
Corrosion
What Is Corrosion?
Corrosion is the breakdown of metals due to reactions with the environment. The most common form is rusting, where iron reacts with oxygen and water to form iron oxide (rust).
Corrosion Process
Corrosion involves electrochemical cells forming on the metal's surface. Iron (the anode) oxidizes (loses electrons), while oxygen (the cathode) reduces (gains electrons), creating rust.
Conditions for Corrosion
Corrosion happens faster in salty water because it increases conductivity, making oxidation and reduction occur more easily.
Protection from Corrosion
Måla: Paint creates a protective layer that keeps out water and oxygen, preventing rust.
Legering: Mixing metals, like stainless steel (iron + chromium), makes them stronger and rust-resistant.
Olja: Oil protects metal by keeping moisture and oxygen away, preventing rust.
Coatings: Protective layers, like paint or zinc coating (galvanization), stop rust by blocking water and oxygen.
Anodizing: A process that forms a strong protective layer on metals like aluminum, preventing rust and improving appearance.
Elektrolys och Användning (Electrolysis and Its Uses)
Electrolysis: The process that uses electricity to drive chemical reactions that wouldn't normally occur. For example, copper ions in copper sulfate solution can be reduced to form copper metal.
Applications:
Metal Extraction: Electrolysis is used to extract metals from ores, like aluminum from bauxite ore.
Electroplating: A process where a thin layer of metal is deposited on the surface of another material, often for improving appearance or preventing corrosion.
Water Splitting: Electrolysis of water splits water into its elements, producing hydrogen and oxygen gases. This can be used for hydrogen fuel production.
Batteries and the Environment
Environmental Concerns: Batteries can contain harmful metals like mercury and cadmium, which can cause pollution if not disposed of properly.
Recycling: Recycling helps recover valuable metals such as lithium and nickel, reducing the need for new mining and preventing pollution.
Alternative Energy: Ongoing research is focused on developing more efficient and environmentally friendlybatteries, like those using sodium or organic materials, to reduce environmental impact
Alloys: Mixtures of metals to improve properties
Steel = Iron + Carbon → Stronger, harder, used in buildings and tools.
Stainless Steel = Iron + Chromium + Nickel → Rust-resistant, used in cutlery and medical tools.
Bronze = Copper + Tin → Corrosion-resistant, used in statues and coins.
Brass = Copper + Zinc → Strong, corrosion-resistant, used in instruments and fittings.
Aluminum Alloys = Aluminum + Magnesium/Silicon → Lightweight but strong, used in airplanes and cars.
Preventing Corrosion with Oxide Layers
Aluminum Oxide (Al₂O₃): Forms a protective layer on aluminum, preventing further corrosion.
Chromium Oxide (Cr₂O₃): Creates a layer on stainless steel, protecting it from rust.
Titanium and Zinc: These metals also form protective oxide layers, protecting them from corrosion.
Most to least reactive metal
1. Magnesium (Mg) – Most reactive
2. Zinc (Zn)
3. Iron (Fe)
4. Lead (Pb)
5. Copper (Cu) – Least reactive
If a more reactive metal is placed in a salt solution of a less reactive metal, a reaction will occur where the more reactive metal replaces the less reactive one.
Compare what happens when the two metals, iron and aluminum, "corrode.
forms rust, which is flaky and keeps corroding deeper. Aluminum forms a protective oxide layer, which prevents further corrosion.