5: reactive intermediates
1/134
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
135 Terms
how can concerted vs non-concerted reactions be differentiated?
concerted = 1 TS; NO intermediate
non-concerted = >1 TS; intermediate(s)
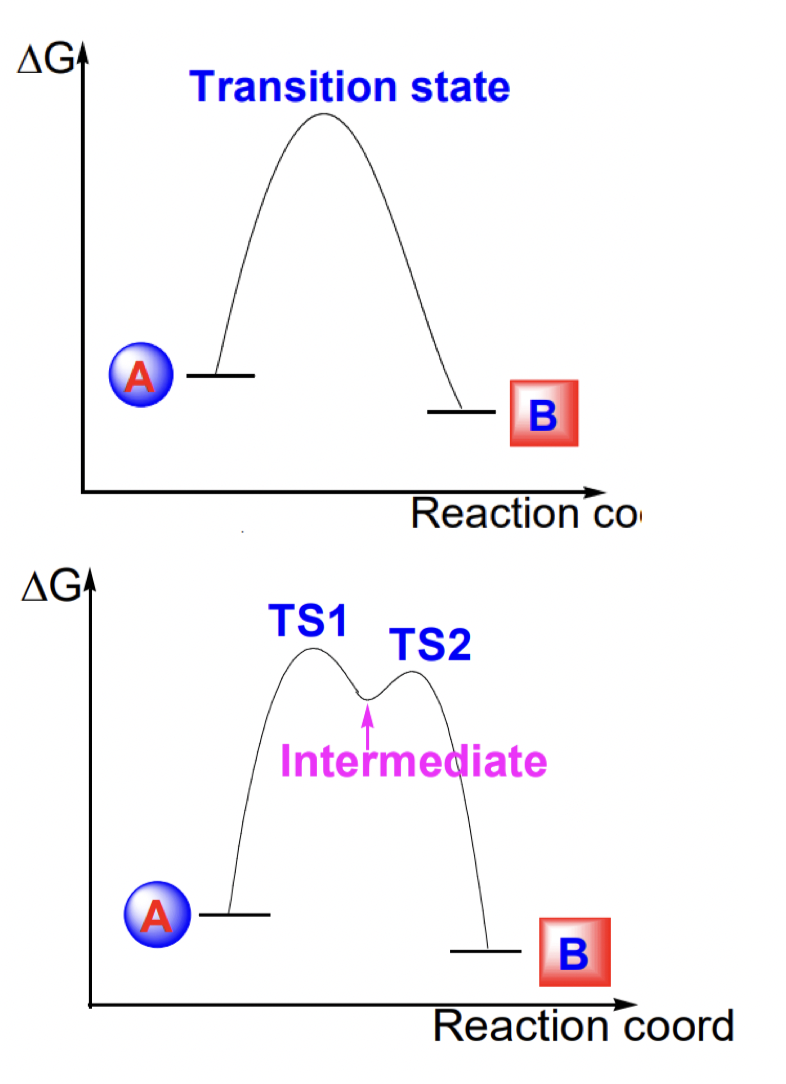
define transition state vs intermediate
TS = “no'“ lifetime (actually femtoseconds) = the highest energy point on the reaction pathway
intermediates = lifetime = in an energy ‘well’
describe the reactivity of intermediates
intermediates are more reactive/unstable than starting material
= cannot be isolated
what are examples of reactive intermediates?
anion
cation
radical
carbene
describe carbenes
neutral sp2 hybridised (2 bonds) carbon with 2 non-bonding electrons
singlet = lone pair in sp2 orbital
triplet = one electron in sp2 orbital; one electron in p orbital
describe radicals
unpaired electrons
organic radicals:
unpaired e(-) typically occupies the unhybridised p orbital perpendicular to plane of sp2 orbitals = SOMO
generally unstable
describe the stages of a radical reaction
radical generation / initiation
radical modification / propagation (may be >1 step)
radical capture / termination
describe radical creation
requires bond homolysis = electrons in a bond move separately = two radicals
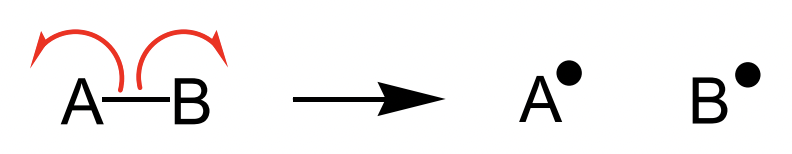
what is a major problem during radical propagation?
dimerisation
radicals are very unstable and tend to forms bond with other radicals
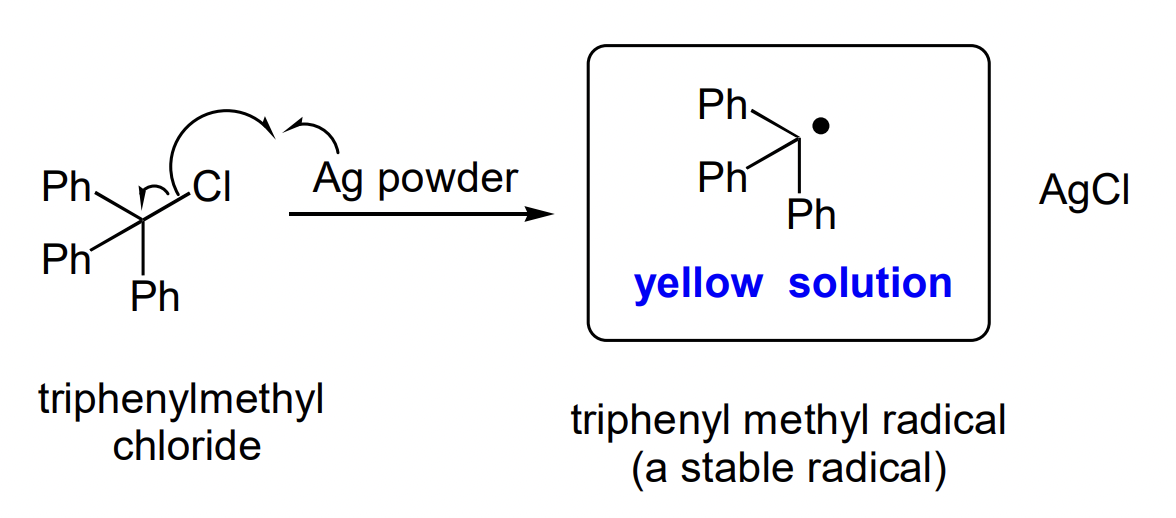
describe the first organic radical
Ag has high affinity for Cl
Al-Cl is very stable
triphenyl methyl radical shows no dimerisation due to steric hinderance
describe stabilisation of organic radicals
any factor which can stabilise cation/anion can also stabilise a radical centre:
conjugation (electronic effects)
EWG (electronic effects)
EDG (electronic effects)
steric effects
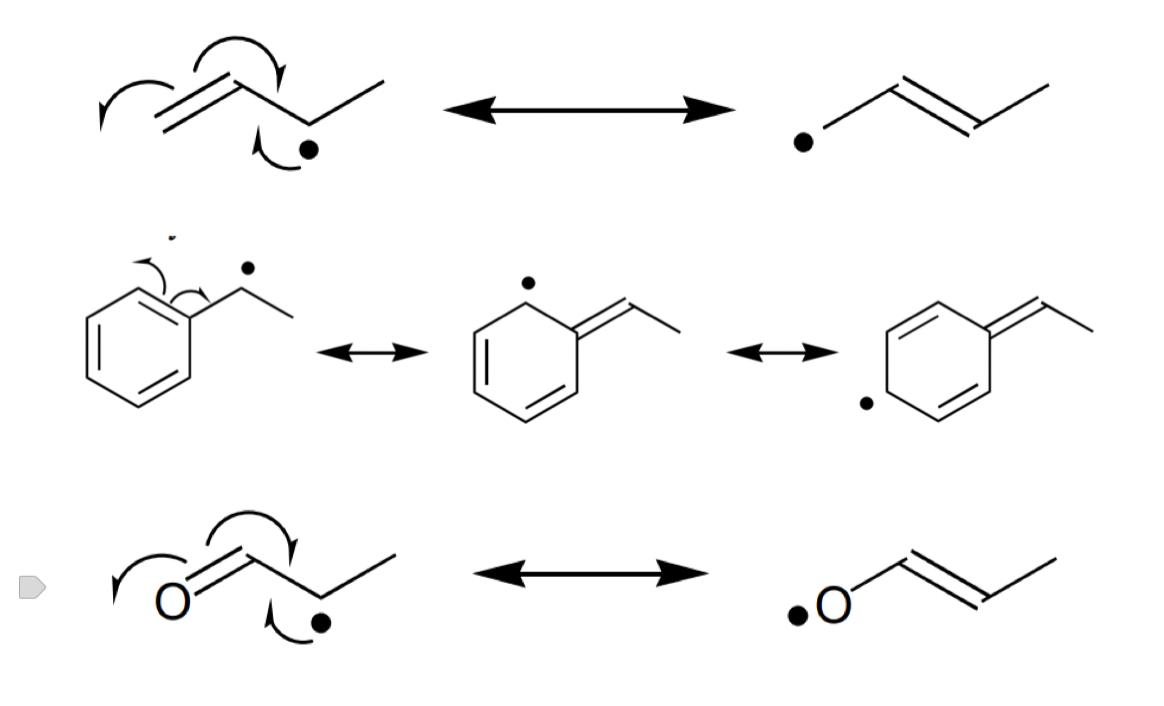
describe stabilisation by conjugation
effective as ∏ and ∏* adjacent to SOMO
possible in:
allylic radical
benzylic radical
radical a to a carbonyl = ∏ too stable due to CO bond stability to interact with SOMO = ∏* more important
describe stabilisation by EDG/EWG effects
EDG = destabilising
EWG = stabilising
describe stabilisation by steric effects
steric protection moderates radical reactivity
very stable radical often require steric AND electronics
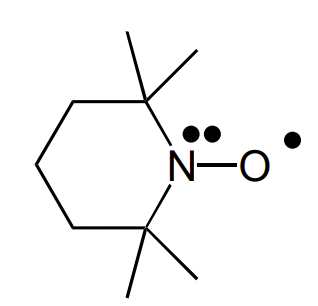
describe the stabilisation of the TEMPO radical
electronic = lone pair in vertical sp3 orbital conjugated to radical
sterics = alkyl ring and alpha methyl groups
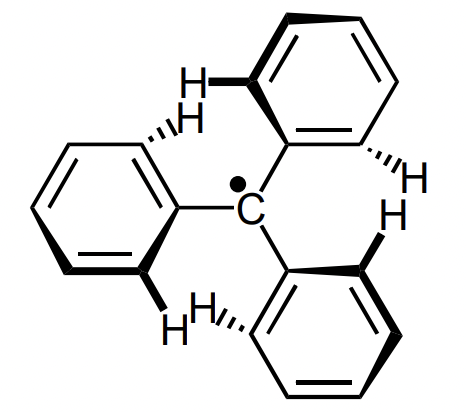
describe the stabilisation of triphenyl radical
electronic = hyperconjugation from alpha C-H’s = requires planarity
sterics = exists in twisted form to avoid clashing H’s = “propeller” shape provides steric hinderance
describe EPR (electron paramagnetic resonance)
electrons have spin of 1/2. unpaired electron spin interacts with the spin of neighbouring protons. applies only to unpaired electrons as pairs cancel out.
coupling gives rise to hyperfine splitting of spin substates
EPR coupling = NMR coupling
couples to protons since have the same spin (1/2)
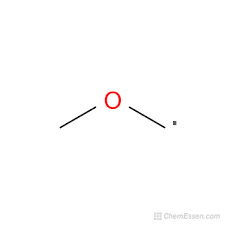
what would the EPR spectrum of methoxymethyl radical look like?
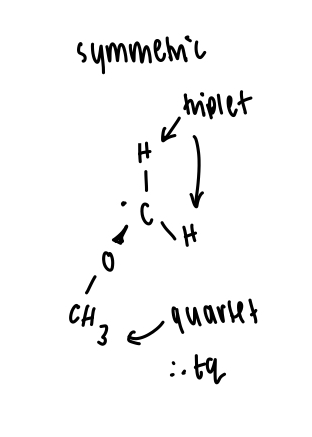
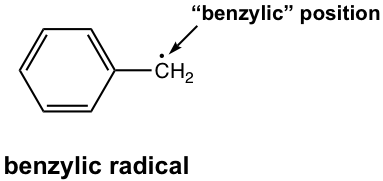
what would the EPR spectrum of benzyllic radical look like?
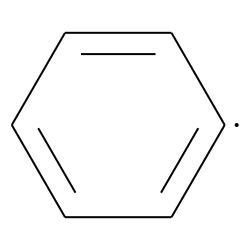
what would the EPR spectrum of phenyl radical look like?
unpaired electron in sp2 orbital

what is BDE?
bond dissociation energy = energy required to achieve homolytic fission in the gas phase
describe the relationship between BDE and:
bond strength
ease of radical formation
radical stability
lower BDE = weaker bond
higher BDE = stronger bond
lower BDE = radical more easily formed
higher BDE = radical less easily formed
lower BDE = radical more stable
higher BDE = radical less stable
how can radical be classified by polar effects?
electrophilic radicals = δ+ = neighbouring electron withdrawing group
nucleophilic radicals = δ- = neighbouring electron donating group
what kind of residues will electrophilic/nucleophilic radicals react with?
electrophilic radicals = electron rich alkenes
nucleophilic radicals = electron poor alkenes
what is a possible application of polar effects on radicals?
synthesis of alternating copolymers
during propagation, nucleophilic radical intermediate will be unreactive to electron poor alkene (itself) and react fast with electron rich alkene = alternating
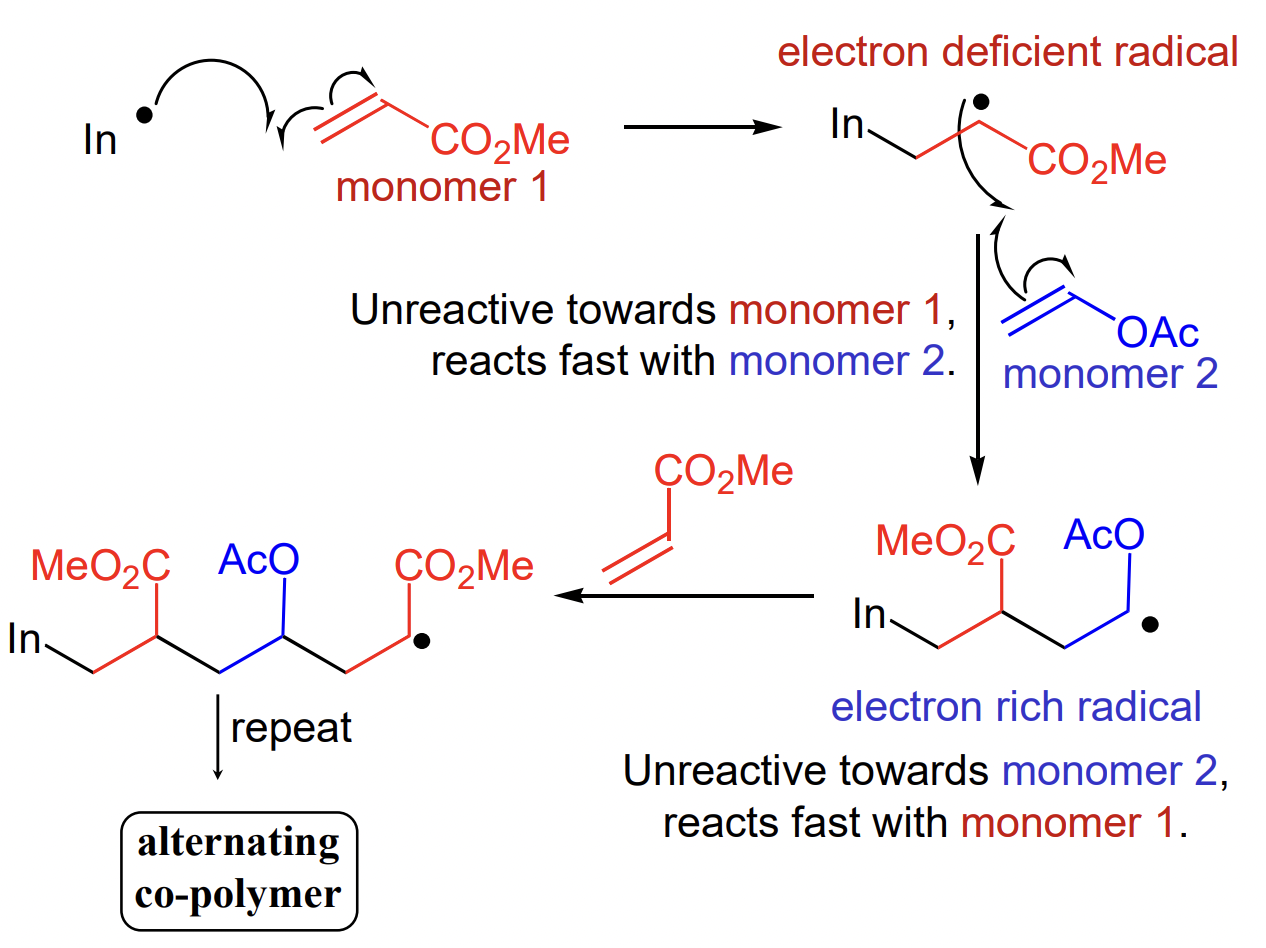
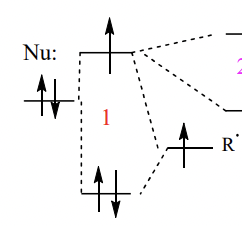
describe the effect of EDG/neighbouring Nu on SOMO + consequence
interaction with lone pair increases the energy of the SOMO
= closer in energy to the empty ∏* orbital of electron poor alkene = preferential interaction
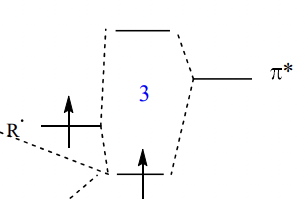
describe the effect of EWG/neighbouring El on SOMO + consequence
interaction with empty ∏* orbital decreases the energy of the SOMO
= closer in energy to the filled ∏ orbital of electron rich alkene = preferential reaction
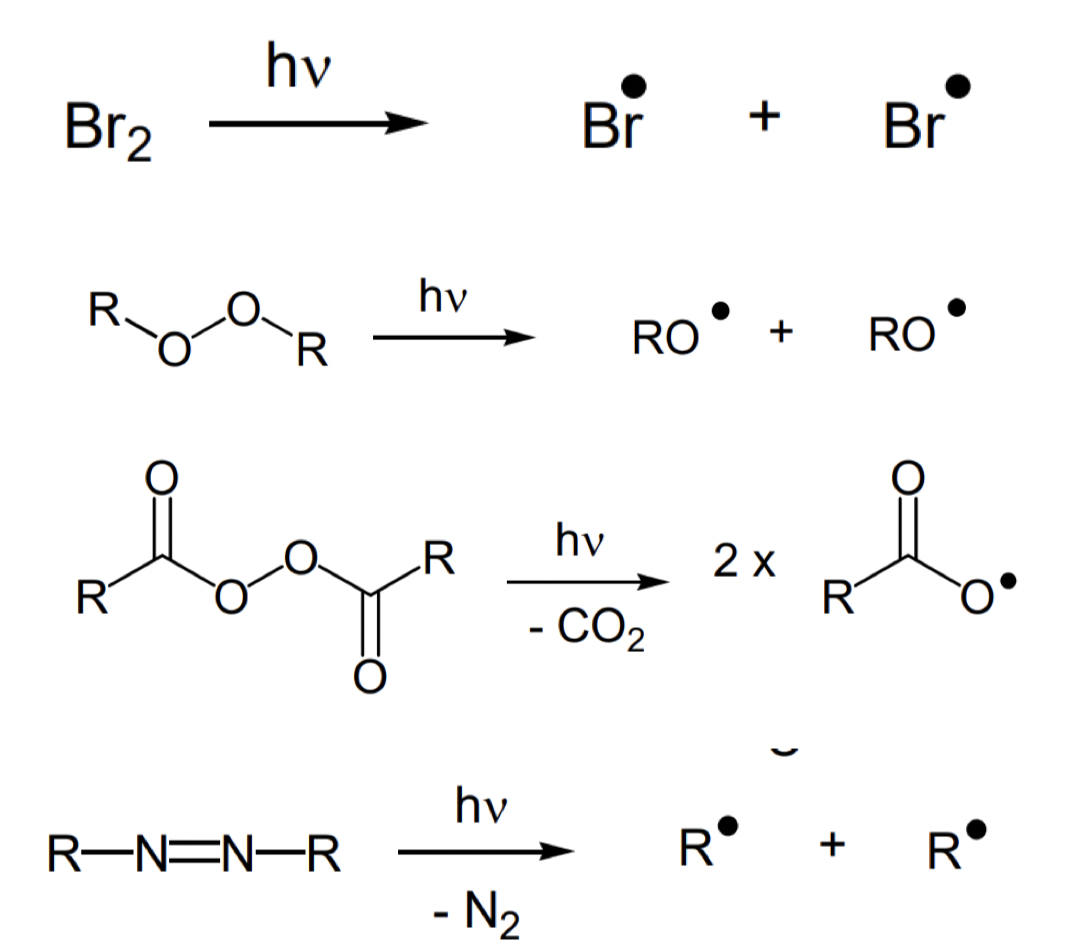
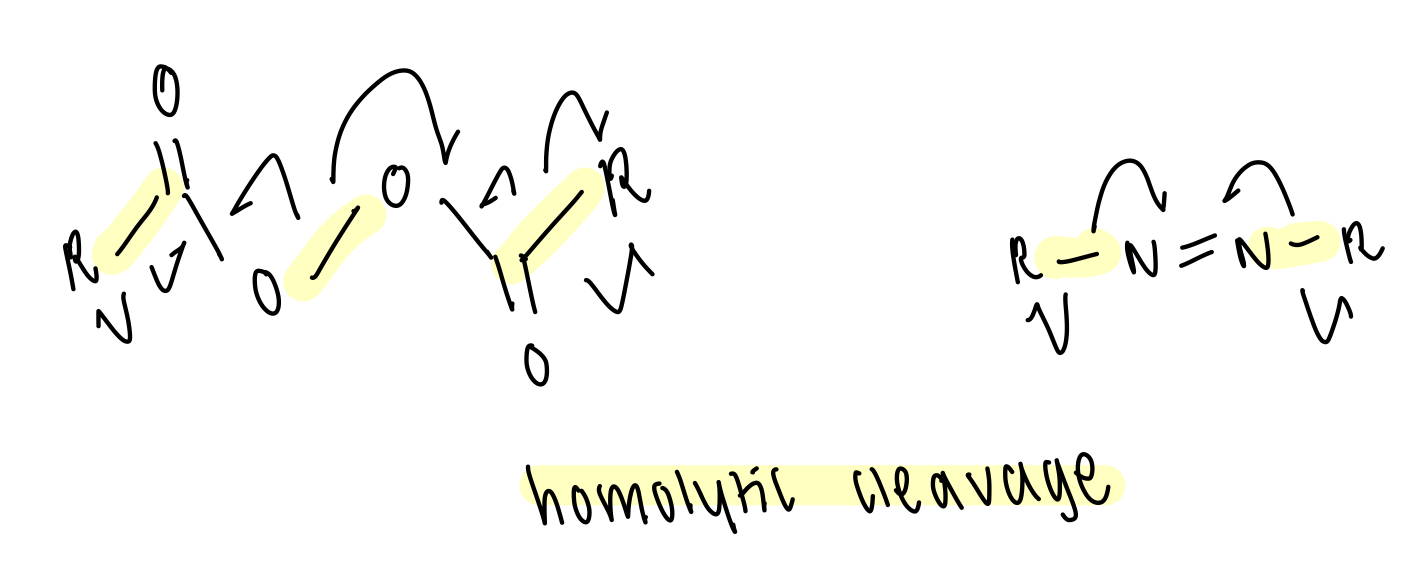
what are methods for initiating radical reactions (radical generation)?
thermal cleavage of weak bonds
thermolysis at elevated temperatures
ideally small BDE (=weak bonds) to minimise temp.
photochemical cleavage of weak bonds
photolysis at room temp
single electron transfer
one electron oxidation/reduction
what compounds readily undergo thermal cleavage?
X-X and X-Y (halides) often weak due to lone pair repulsions
diacyl peroxides
azo compounds
describe thermal cleavage of diacyl peroxides
weaker O-O bond due to resonance stabilisation of TS and products
driven by evolution of gaseous side product
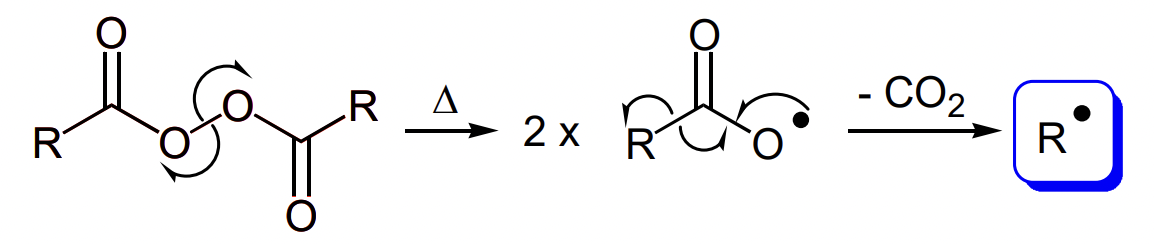
describe thermal cleavage of azo compounds
driven by evolution of gaseous side product
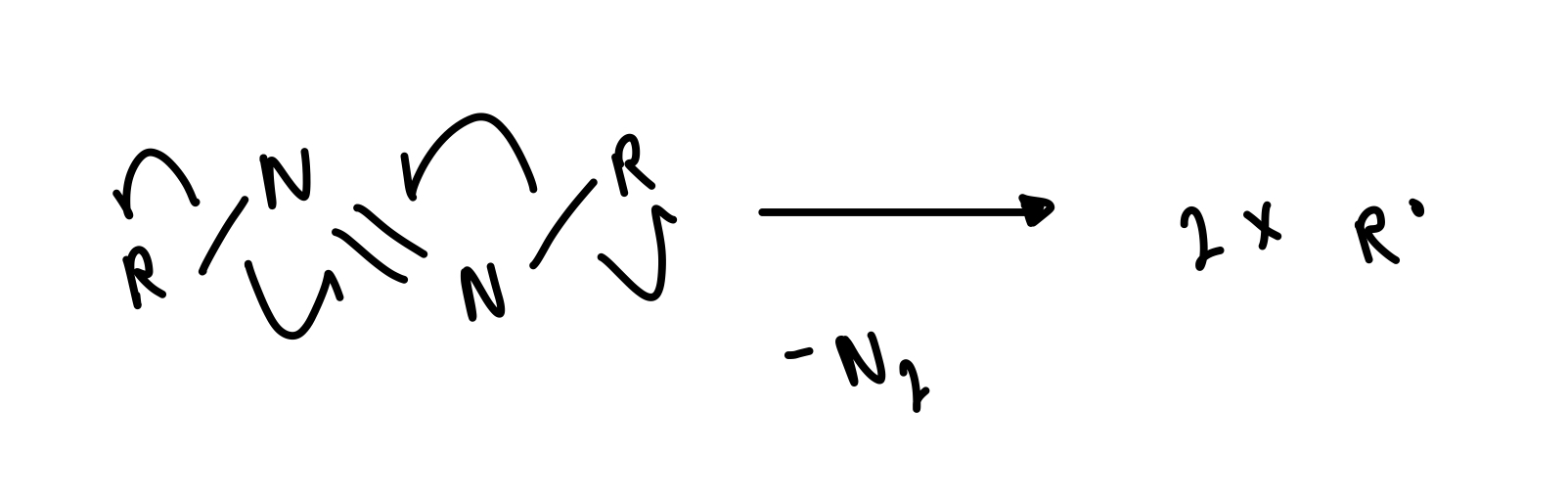
what is one of the most commonly used radical initiators? draw structure and initiation
AIBN
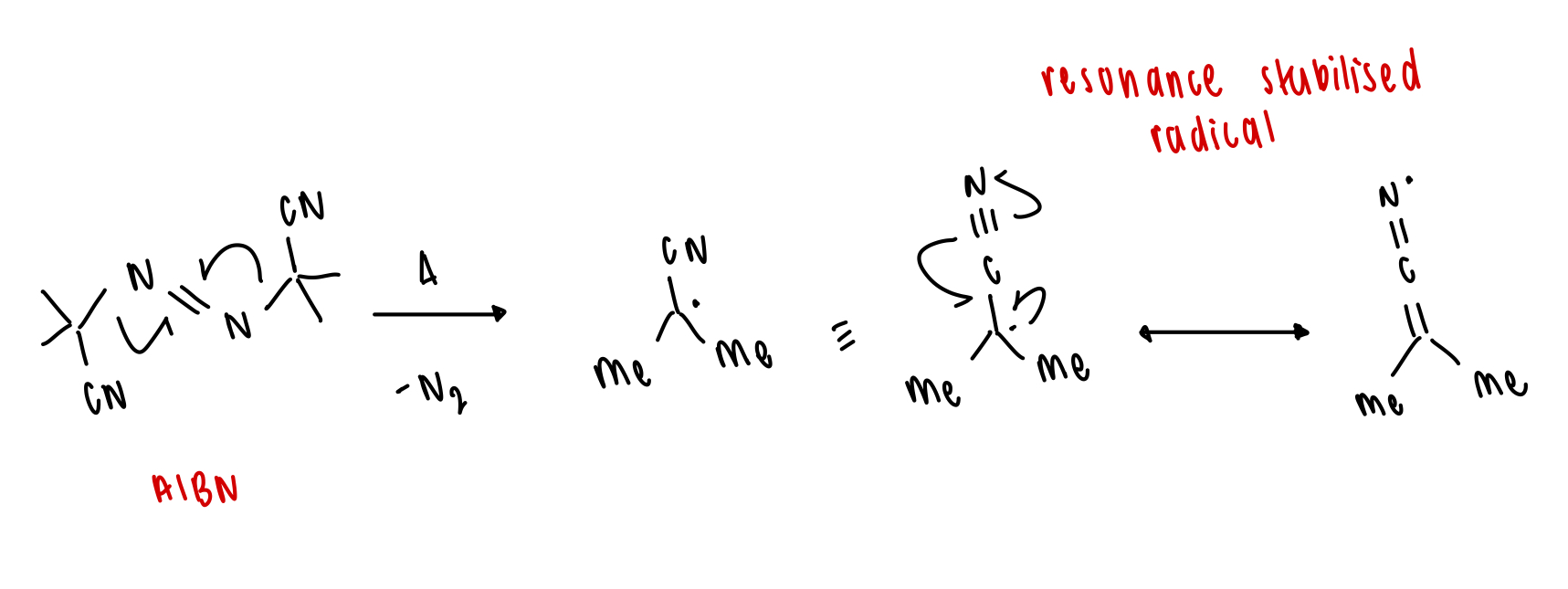
describe photolysis of weak bonds
X-X and X-Y (halides) often weak due to lone pair repulsions
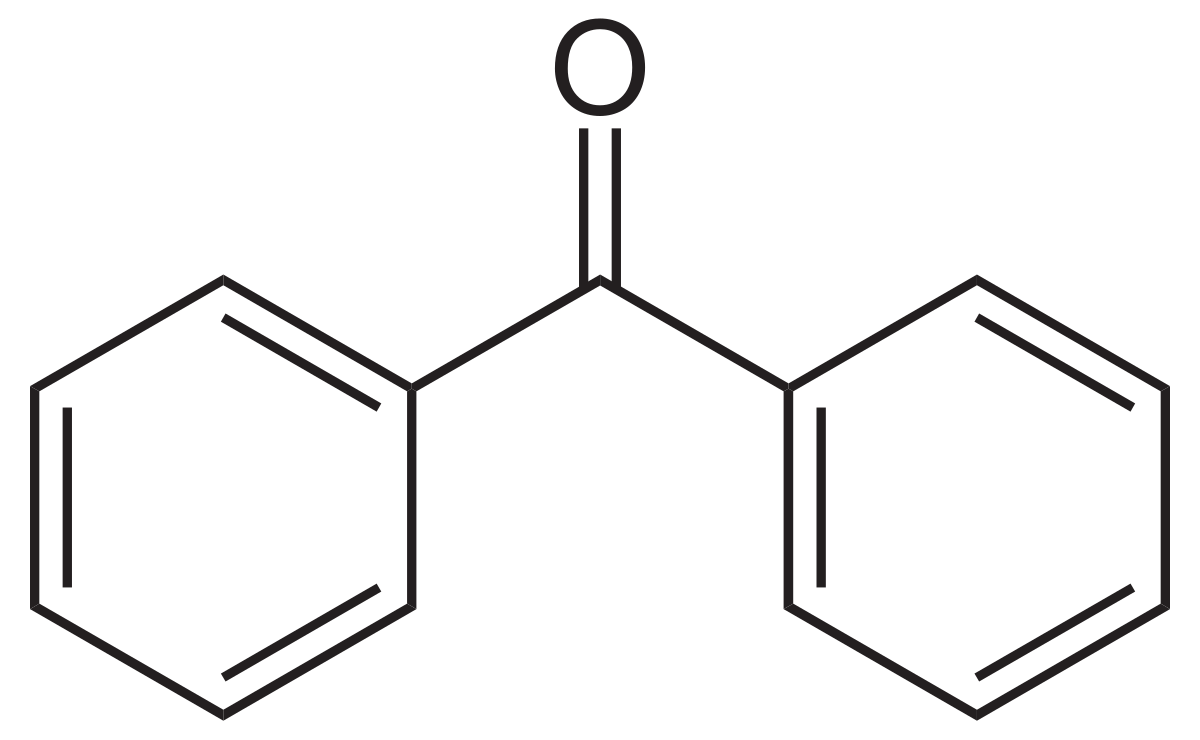
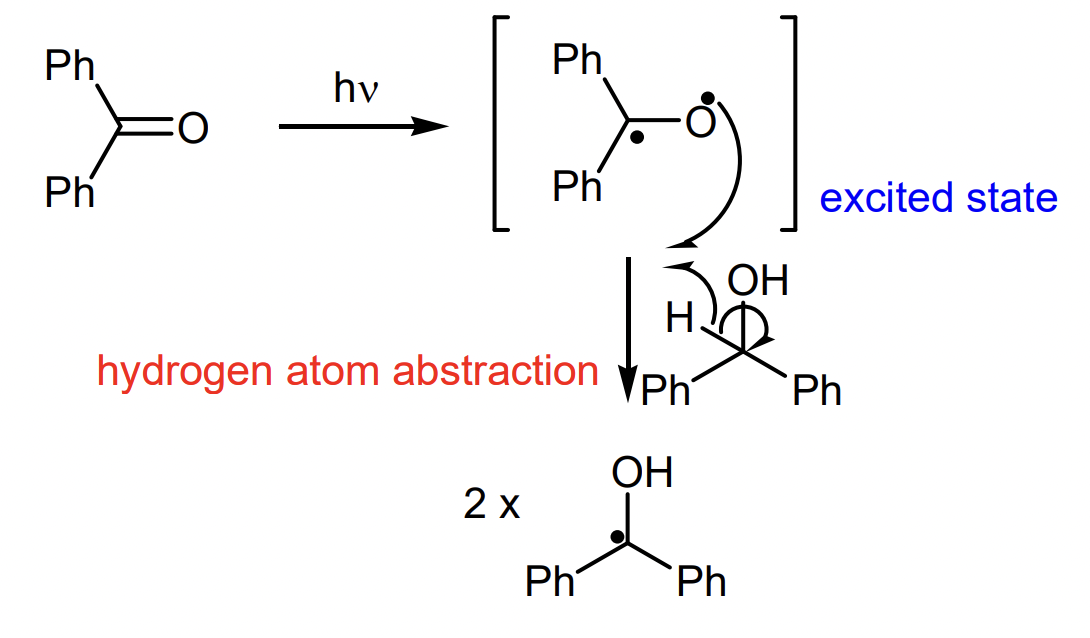
describe what occurs after photolysis of benzophenone
intermolecular atom transfer (can also be intramolecular)
what is the simplest reagent of single electron transfer
dissolving metal (Na/K) in NH3(l):
Na(+)/K(+) and e(-) free in blue solution
what are two types of single electron transfer reactions?
Birch reduction
Samarium iodide
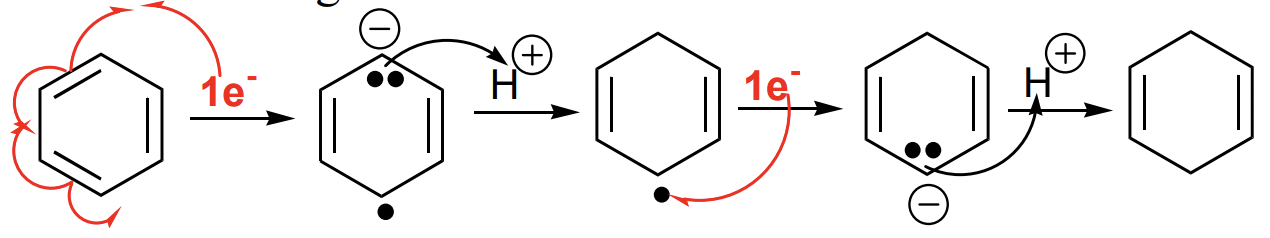
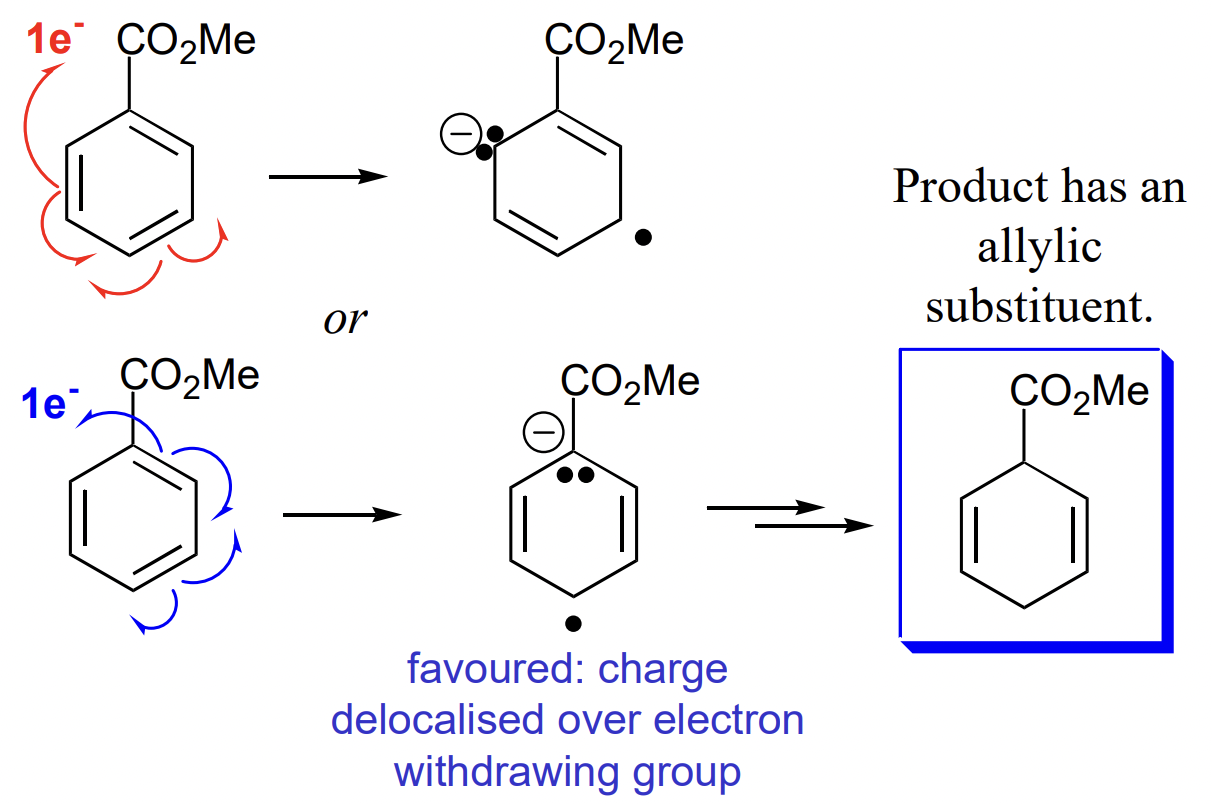
describe the Birch reduction
reduction of aromatic compounds by sequential transfer of e- and H(+) to an aromatic ring
→ non-conjugated cyclohexa-1,4-diene
can be directed by EWG/EDG substituents
describe Birch reduction with EDG substituent
vinylic = attached to double bond
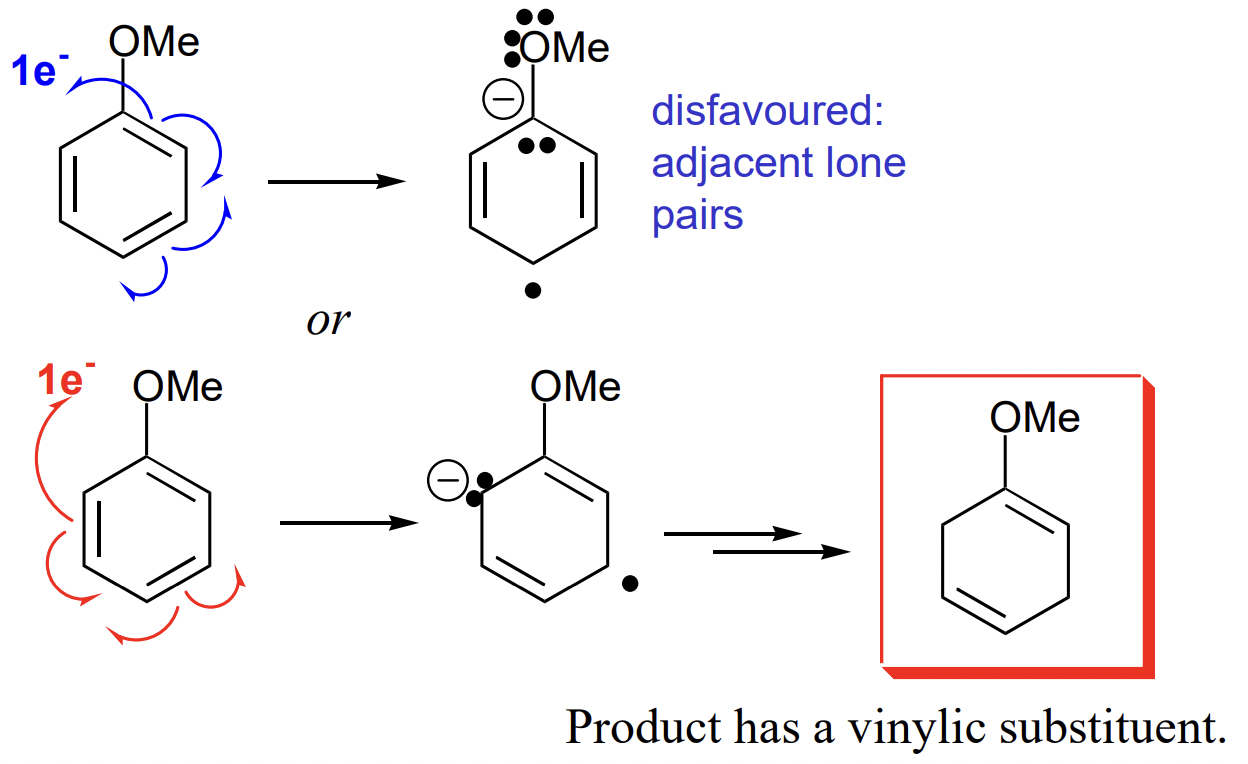
describe Birth reduction with EWG substituent
allylic = adjacent to double bond
*any EWG oxidising on its own i.e. NO2 will be reduced*
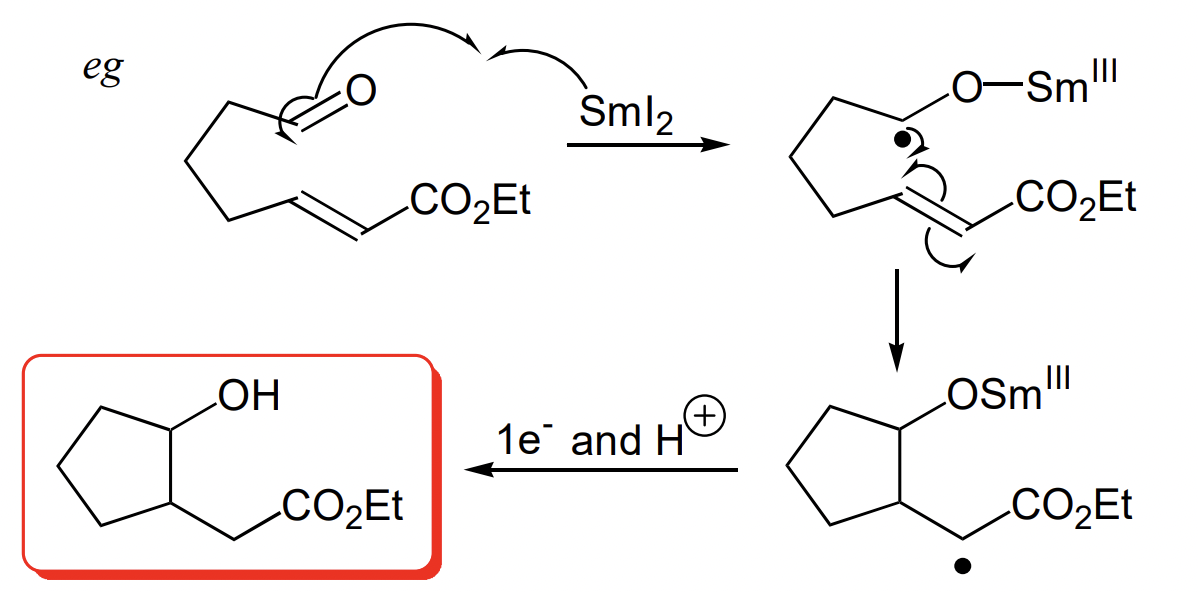
describe single electron transfer with Samarium iodide (SmI2)
SmI2 = lanthanide reagent
Sm can be Sm(II) or Sm(III) = easily give up electron under mild conditions = reducing agent
i.e.
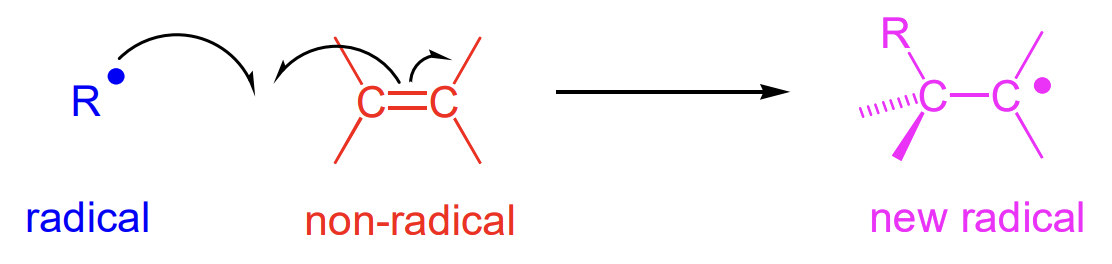
what are two types of radical propagation?
intermolecular reaction
intramolecular reaction
describe types of intermolecular reactions
reaction of radical with a stable molecule
intrinsically slower than combination with another radical
generates new radical
radical addition reactions (i.e. alternating polymerisation)
atom abstraction (radical substitution reactions)
fragmentation reactions

describe radical addition reactions
alkenes are common targets
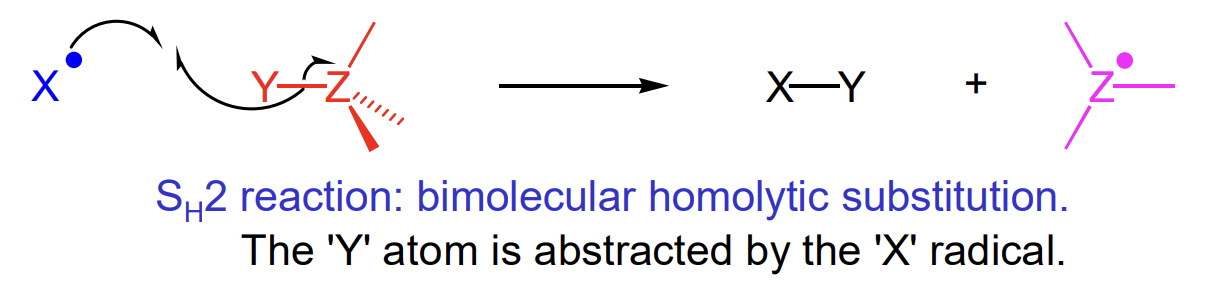
describe intermolecular atom abstraction reactions
radical does an SN2-like attack on abstracted atom
favoured is stronger bond is formed
often Z = C
rarely Y = C
describe fragmentation reactions
i.e. AIBN thermal cleavage
loss of stable molecule
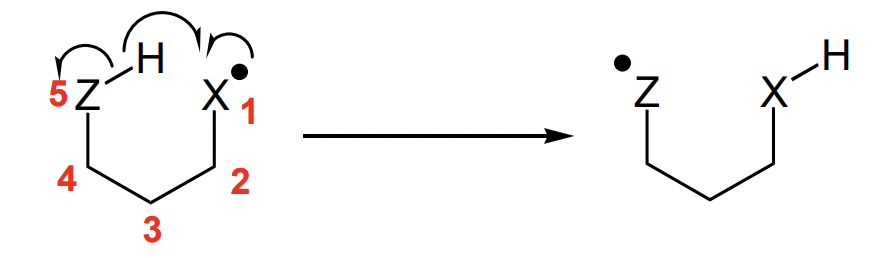
describe types of intramolecular reactions
intrinsically fast
ring closing reaction
intramolecular atom abstraction
ring opening reaction
describe intramolecular ring closing reaction
alkene → single bond (ring)
= intramolecular version of radical addition
describe intramolecular atom abstraction
i.e. 1,5-hydrogen atom abstraction
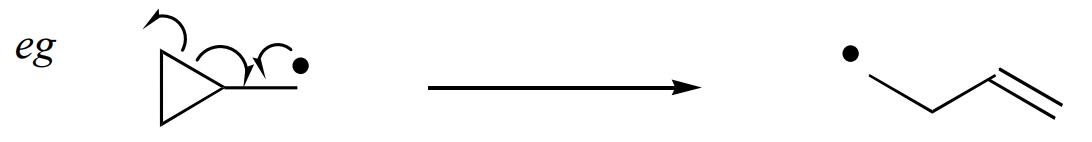
describe intramolecular ring opening reaction
driving force = relief of ring strain
forms alkene = reverse of radical addition
describe termination of radical reactions
typically radical recombination
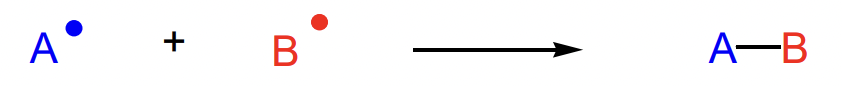
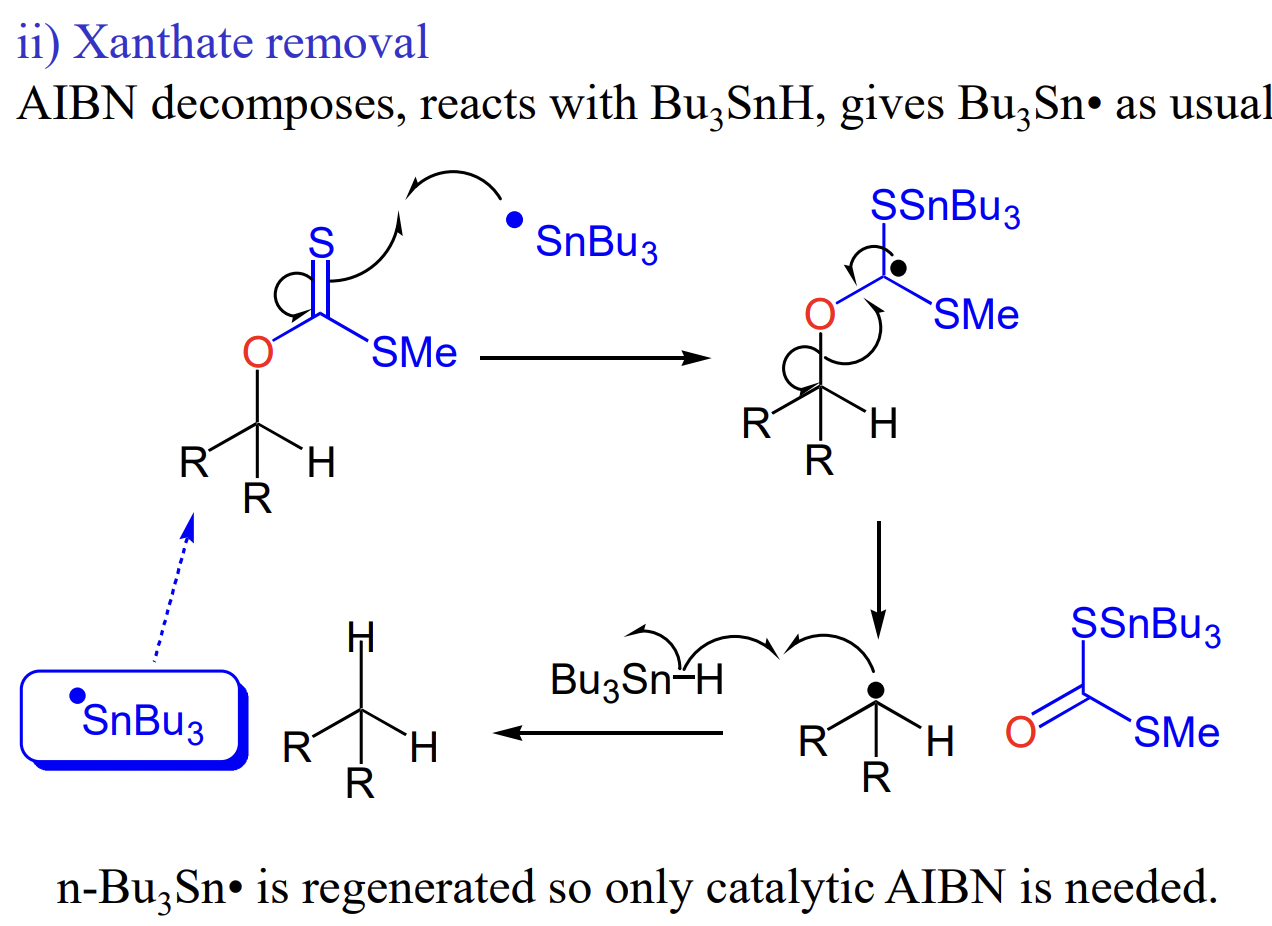
what is the most common initiator and catalyst in radical reactions?
AIBN + Bu3SnH (tributyltin hydride)
weak Sn-H = poor orbital overlap
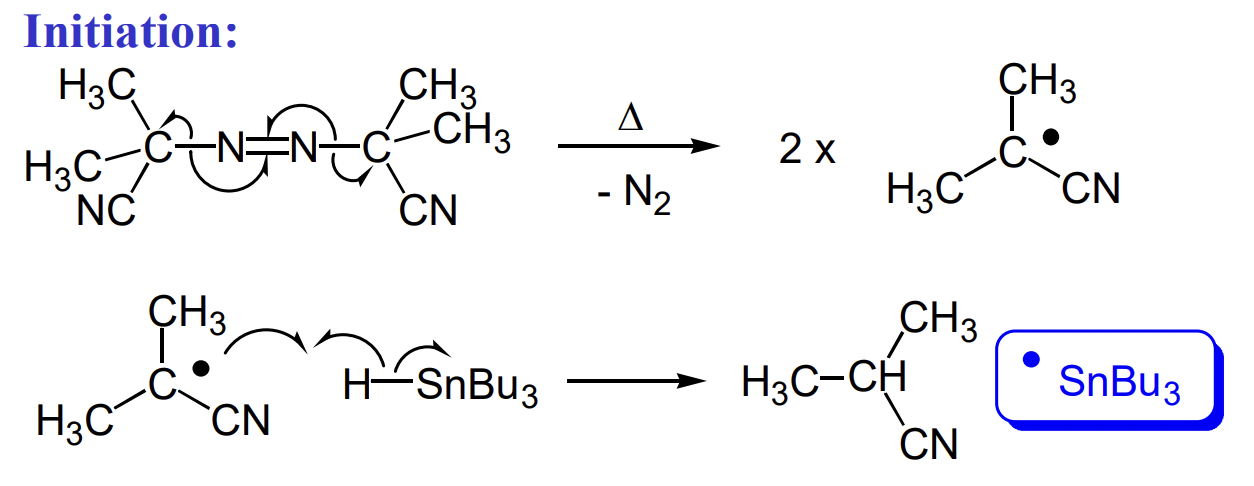
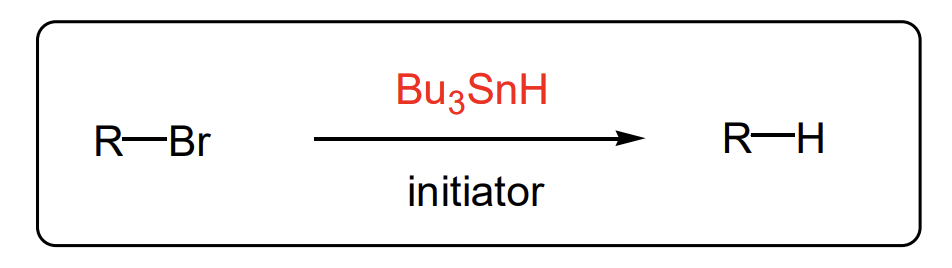
describe the reduction of haloalkanes to alkanes with tin hydride
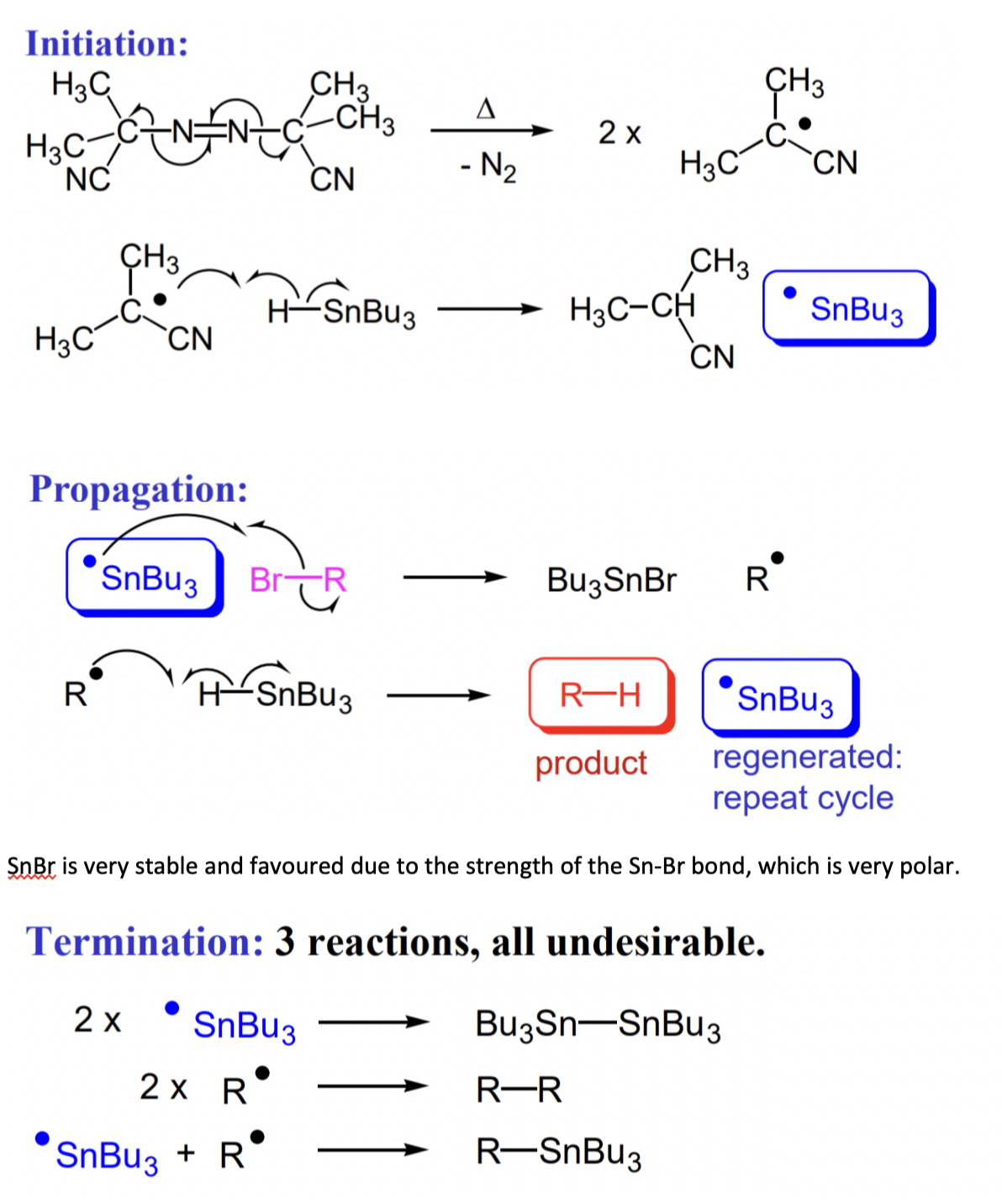
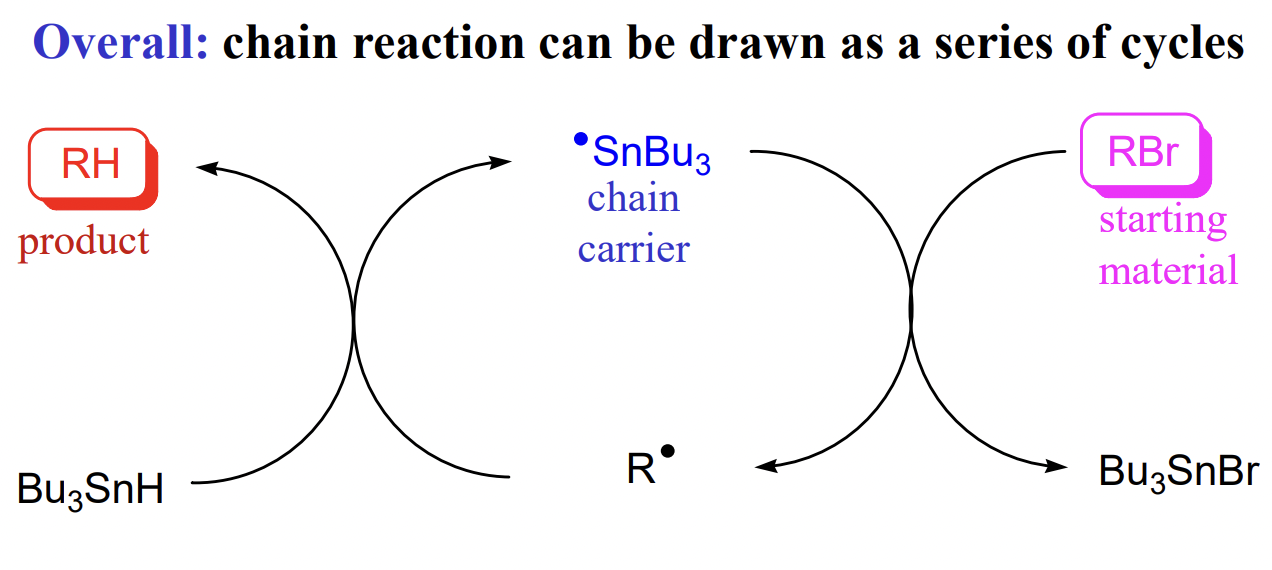
describe an advantage of radical reactions
= compatibility with many functional groups
i.e. O-H bond cleavage in not usually a problem = high BDE
what are three types of radical reactions?
functional group removal
intermolecular addition to multiple (double) bonds
intramolecular addition to multiple (double) bond = cyclisation
what are different types of functional group removal
removal of X from R-X (seen)
removal of OH from R-OH: 2 types
removal of COOH from R-COOH
what is a driving force in many Barton reactions
= strength of S-Sn bond
what are the two types of dehydroxylations?
= both follow Barton-McCombie mechanisms
LG:
xanthate
thiocarbonyl-imidazole
describe Barton-McCombie dehydroxylation
R-OH → R-xanthate → R-H
only for 2° or 3° alcohols
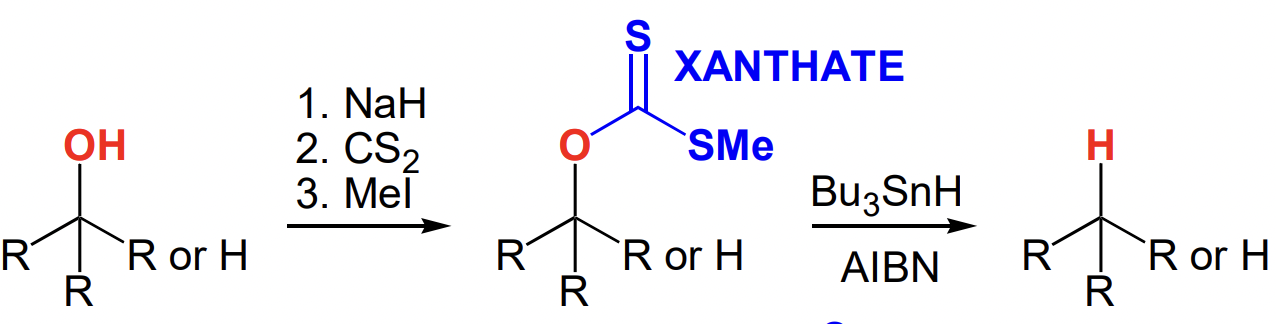
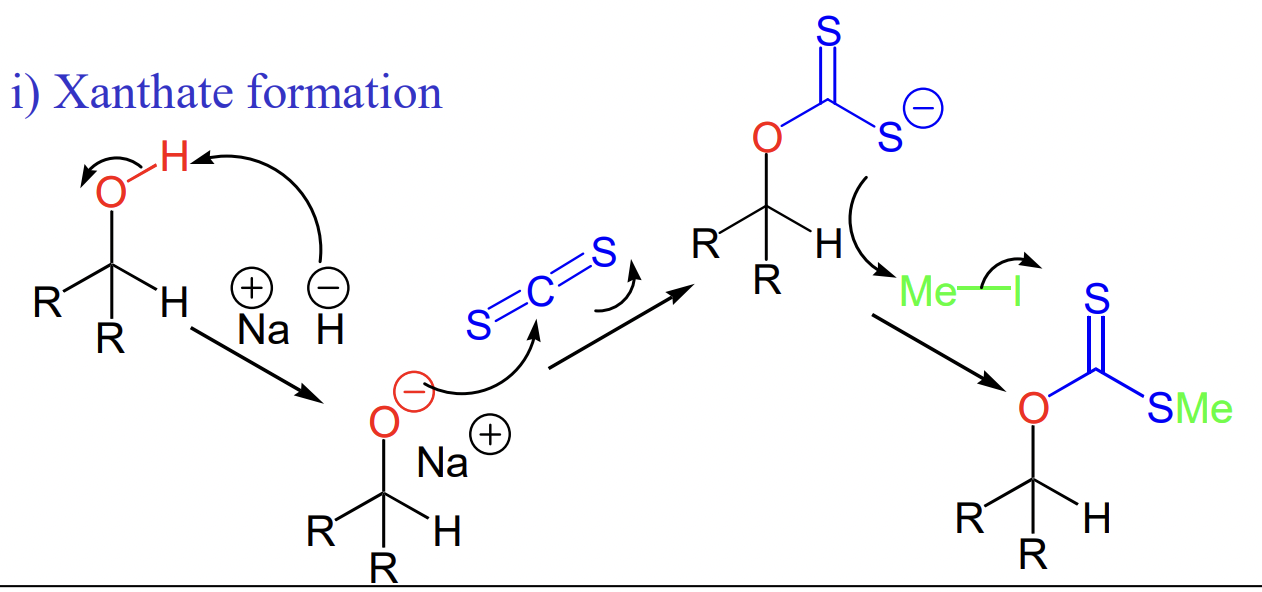
draw xanthate formation
reagents:
NaH
CS2
MeI
draw xanthate removal
AIBN not catalytic = not regenerated = initiator
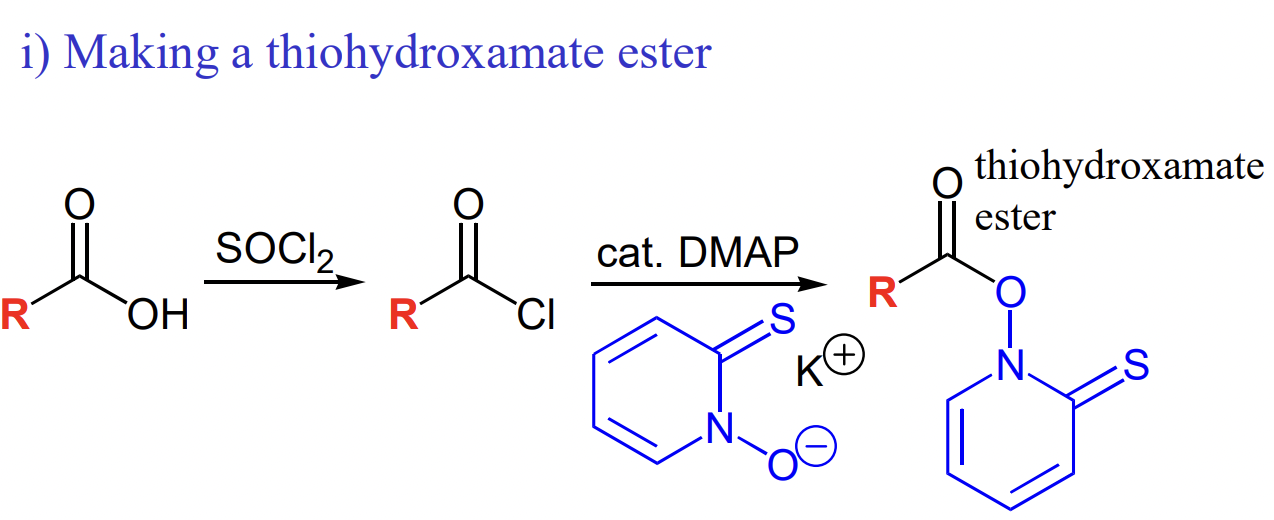
describe Barton decarboxylation
R-COOH → R-COCl → R-CO-thiohydroxamate ester
draw dehydroxylation via thiocarbonyl-diimidazole
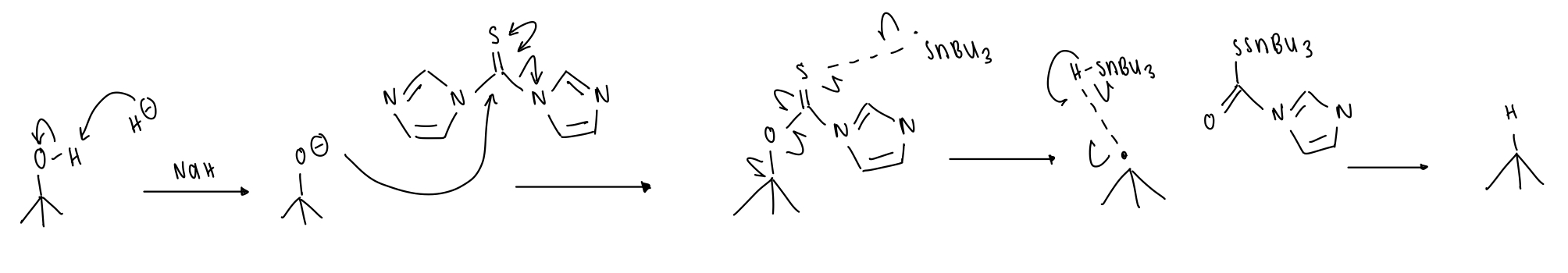
draw the reagents of Barton decarboxylation
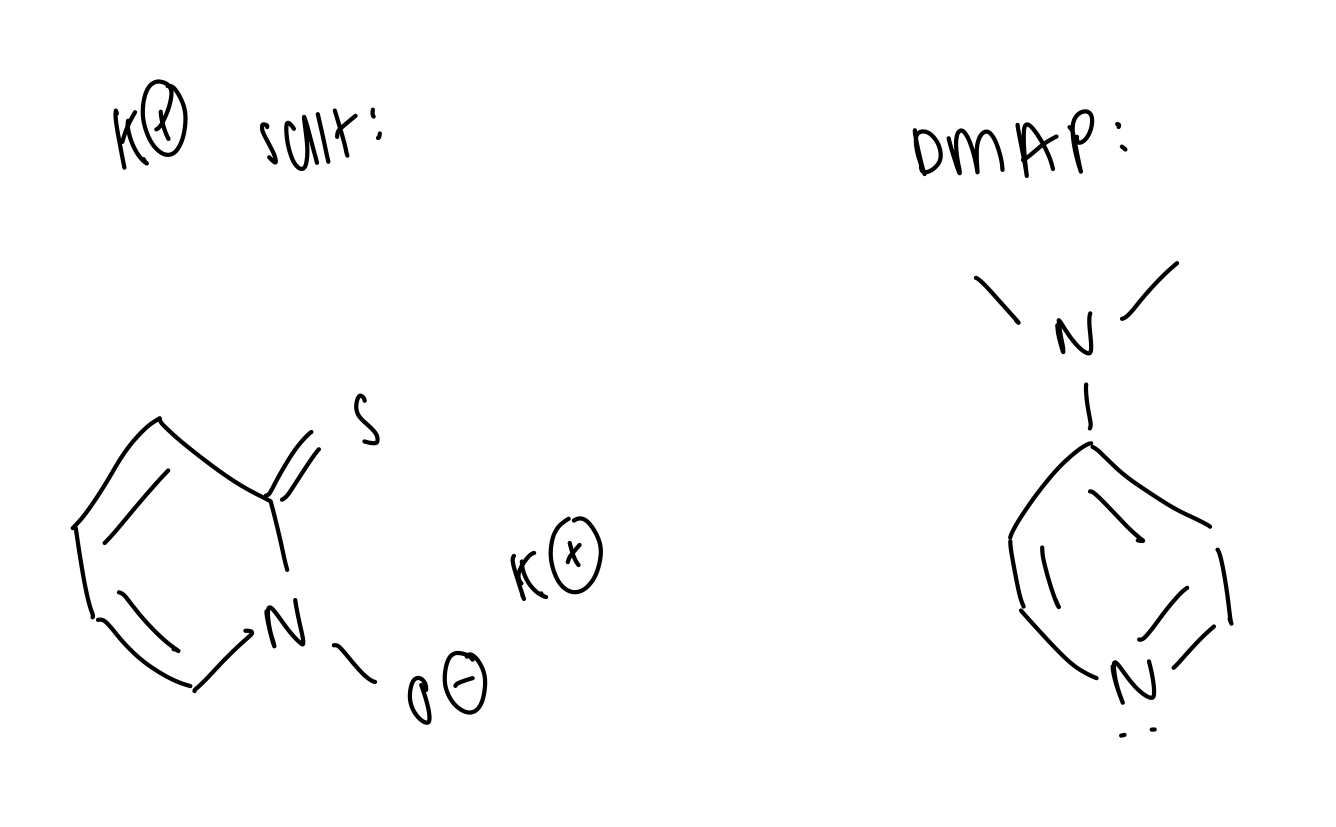
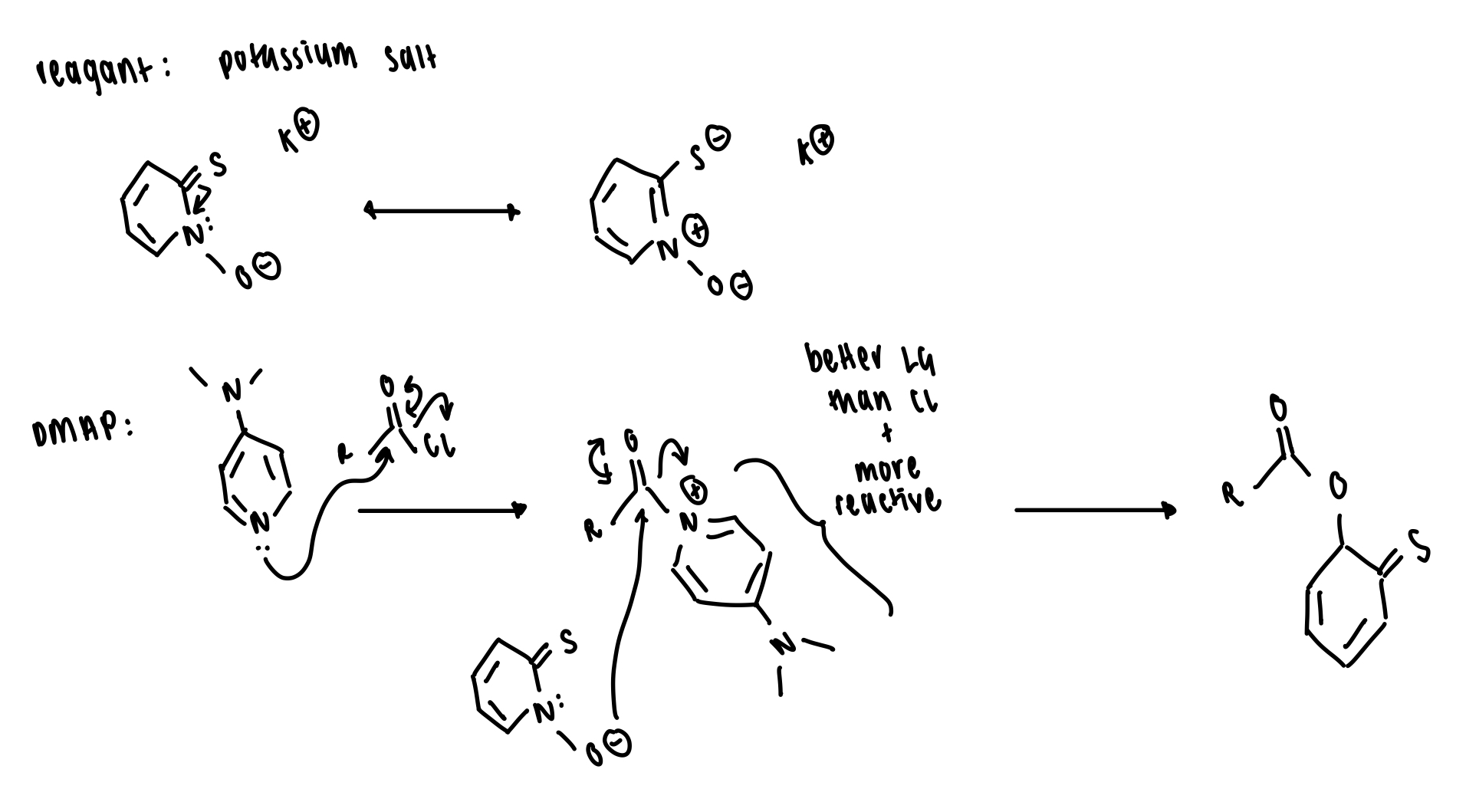
DMAP = to replace Cl and make an even better LG
K salt = to displace the LG
draw the first stage of Barton decarboxylation
= formation of thiohydroxamate ester
draw the second stage of Barton decarboxylation
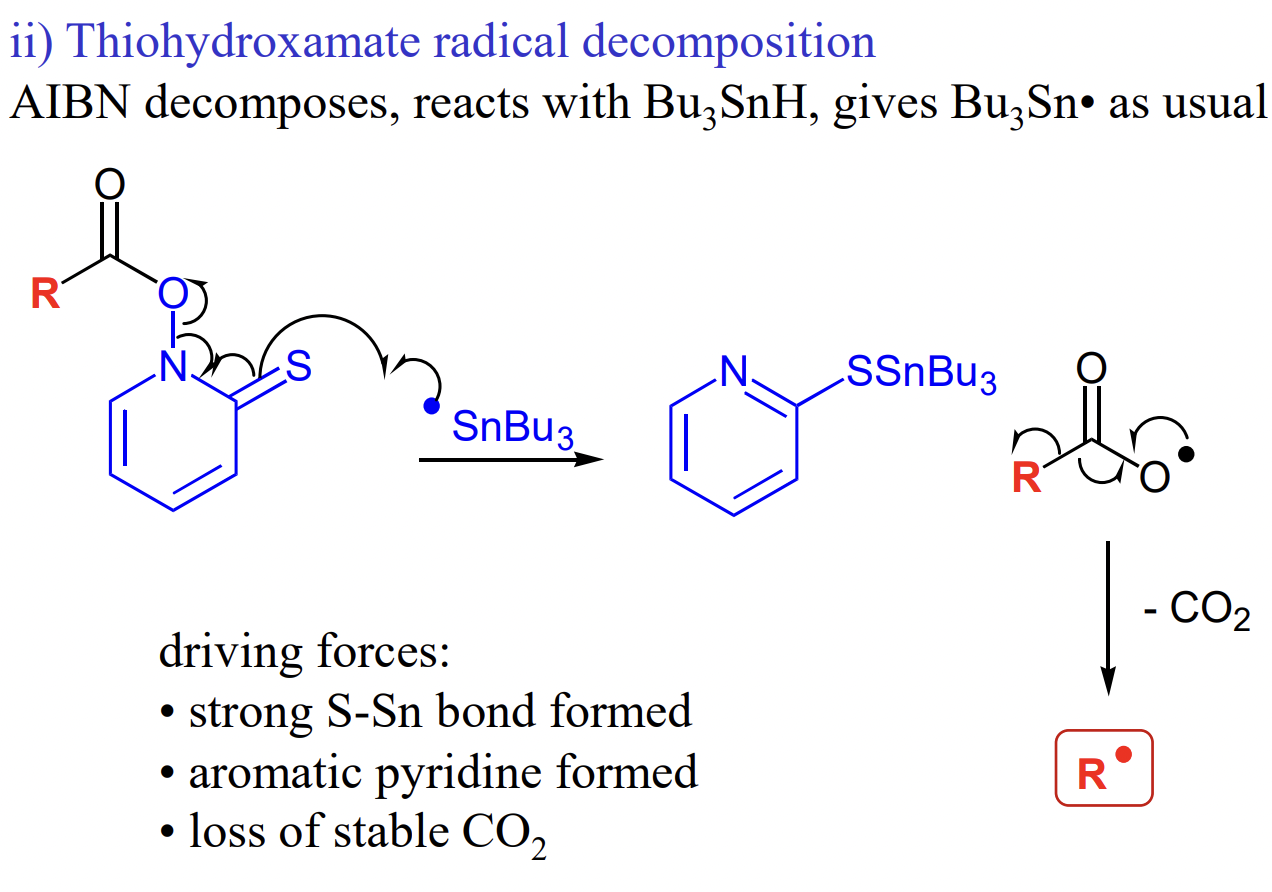
describe intermolecular addition to multiple bonds
radical + alkene → alkane
(here) radical generated by cleavage of R-X bond
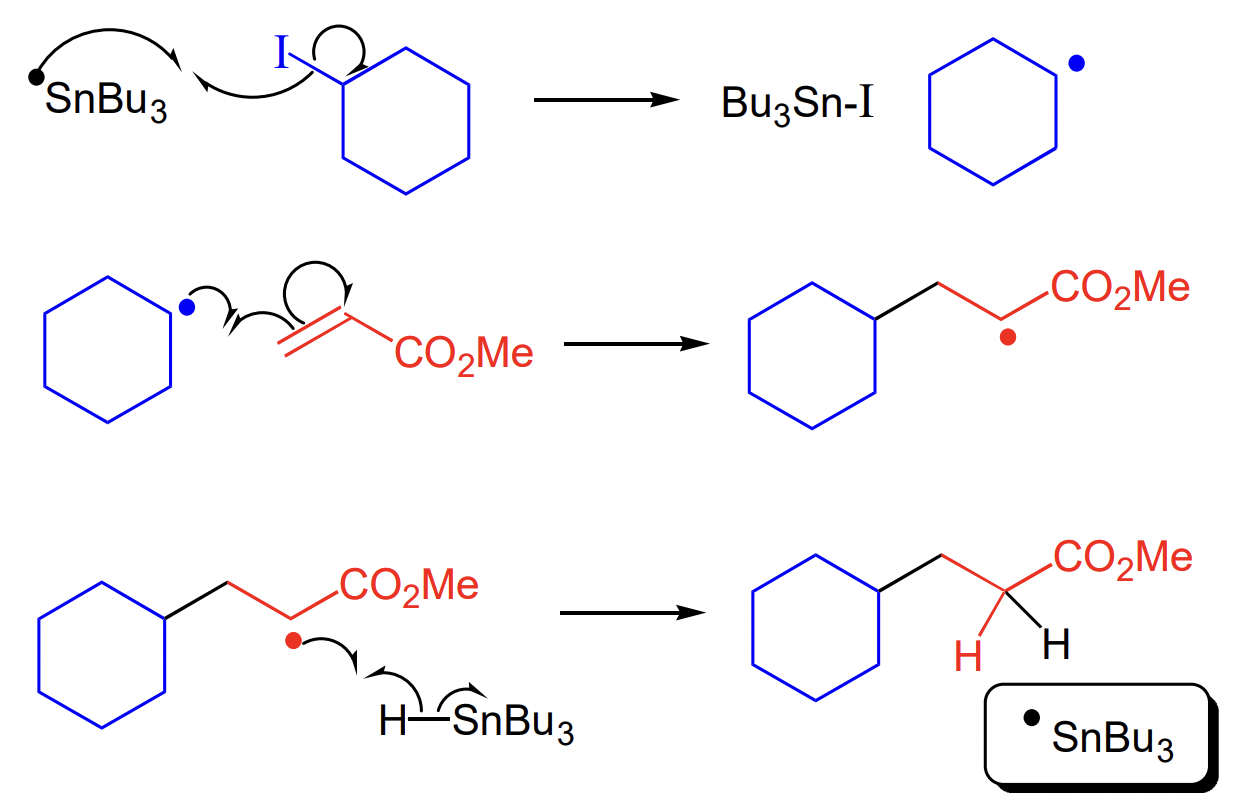
describe intramolecular addition to multiple bonds
radical + alkene → ring alkane
= fast = entropically favoured
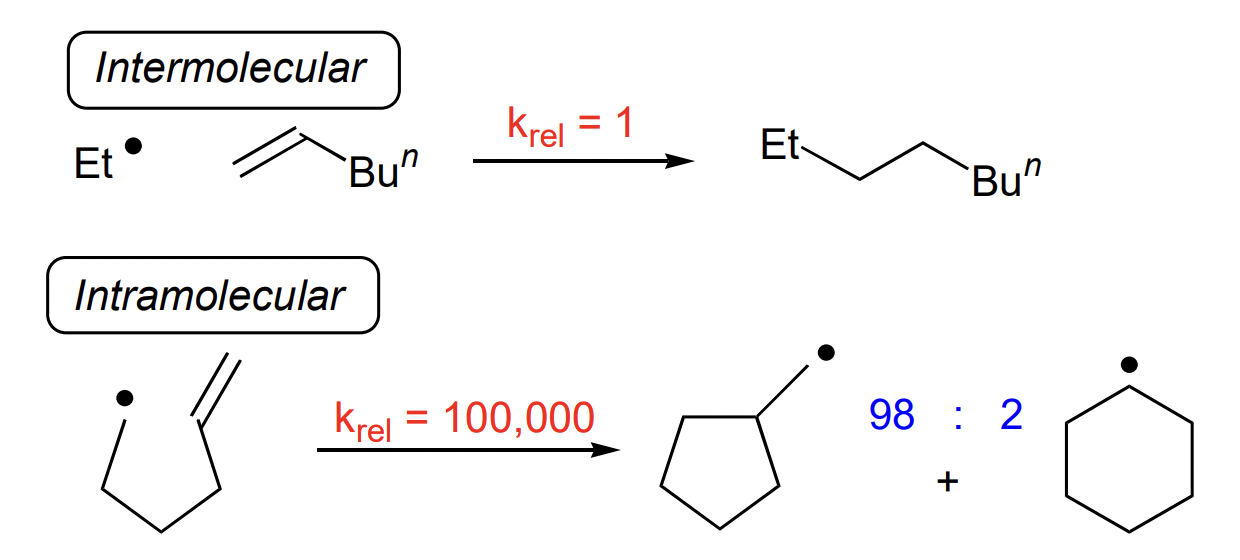
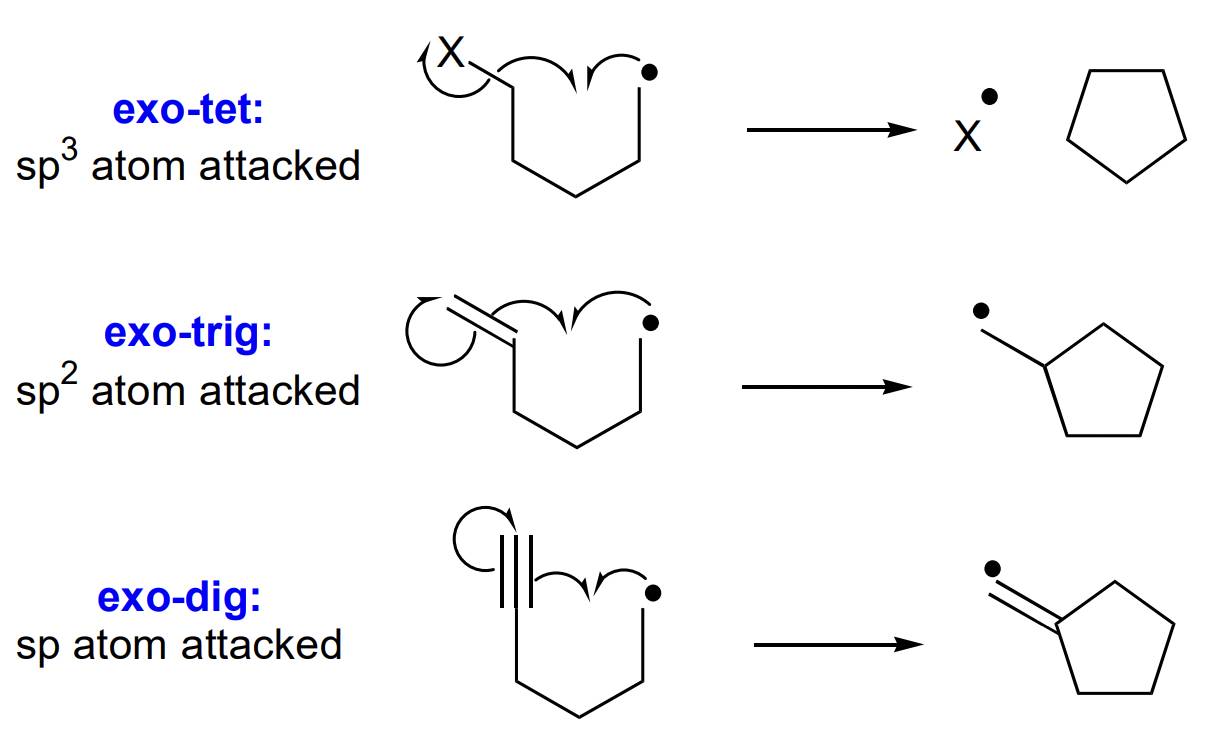
how are ring closures named?
X-exo/endo-trig/tet etc.
X = size of ring
exo = alkene outwith ring of TS
endo = alkene within ring of TS
tet = sp3 atom attacked (alkane)
trig = sp2 atom attacked (alkene)
dig = sp atom attacked (alkyne)
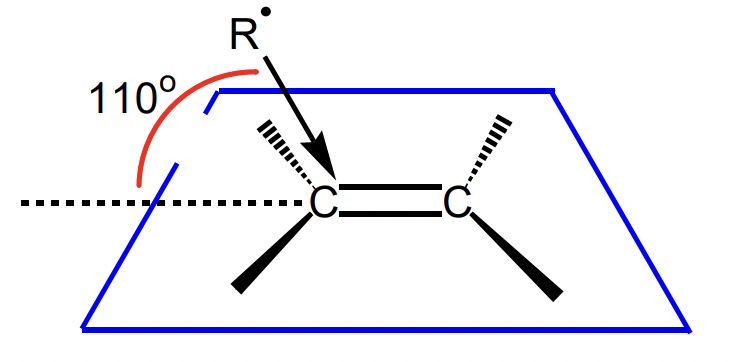
what is the preferred angle of attack on alkenes?
110°
what kind of cyclisation is favoured by radicals?
exo radical cyclisation
what size of ring is favoured by radical cyclisation?
5 > 6 > 3/4 > 7/8
describe the size of the ring with angle of attack
5: exo cyclisation close to preferred angle of attack
at ring size increases, the endo TS angle increases and approaches preferred angle of attack
increasing ring size = increasing proportion of endo product
describe radical clocks
ring opening reactions = rate well known
can set up reaction which have oxidation and cyclisation possible:
oxidation product observed = oxidation faster than cyclisation
cyclisation product observed = cyclisation faster than oxidation
can alter the speed of cyclisation by altering the ring size (size of ring):
ring strain ∝ speed of radical clock
describe an application of radical cyclisation
formation of large rings
what can disfavour a reaction towards radical cyclisation
= unfavourable angle
= favours reduction of radical intermediate instead
how can radical cyclisation be favoured?
using radical reagents with no available H atom
i.e. (Bu3Sn)2 not HSnBu3
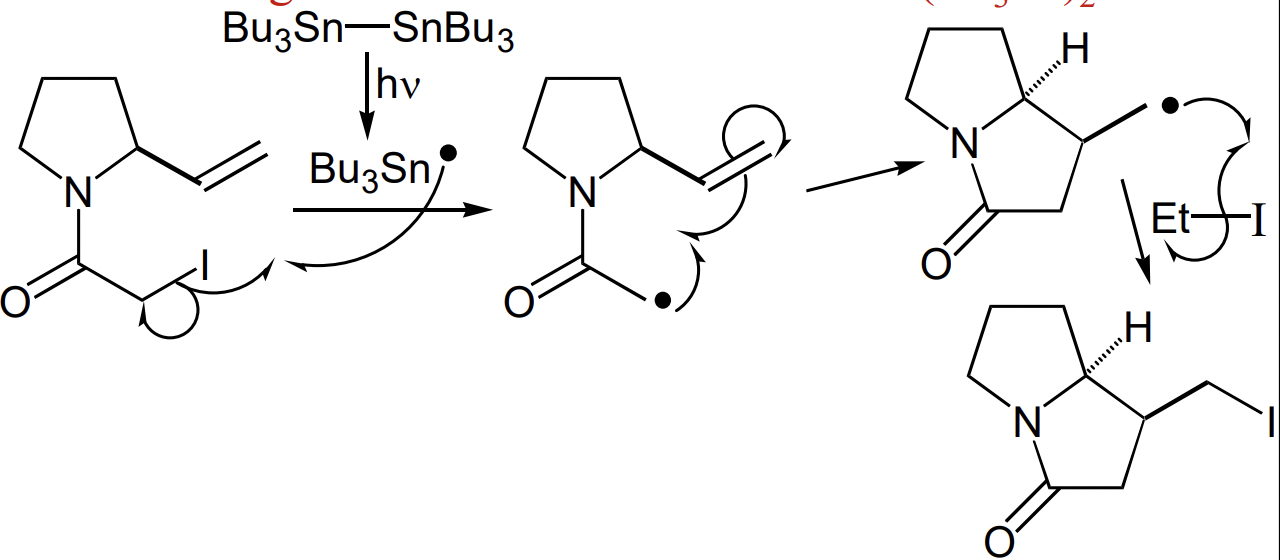
what are sequential/tandem radical reactions?
before radical termination, a radical can undergo multiple reaction steps
i.e.
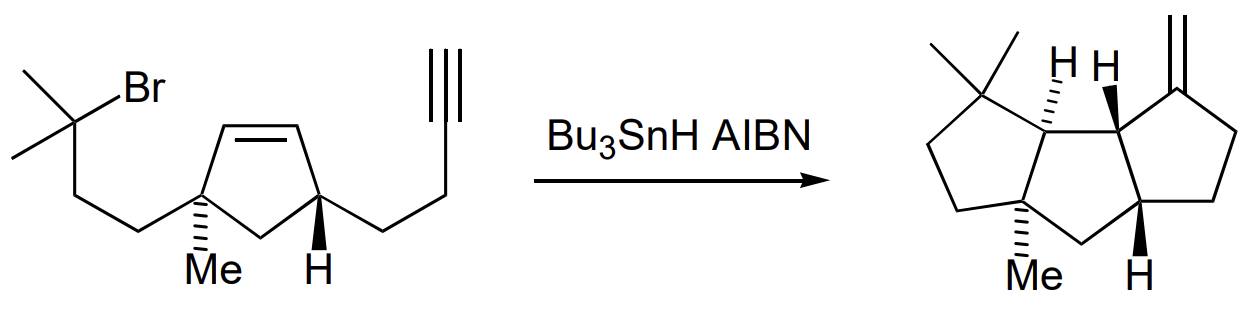
describe metal-mediated radical reactions
= radical reaction initiated by single electron transfer from metal
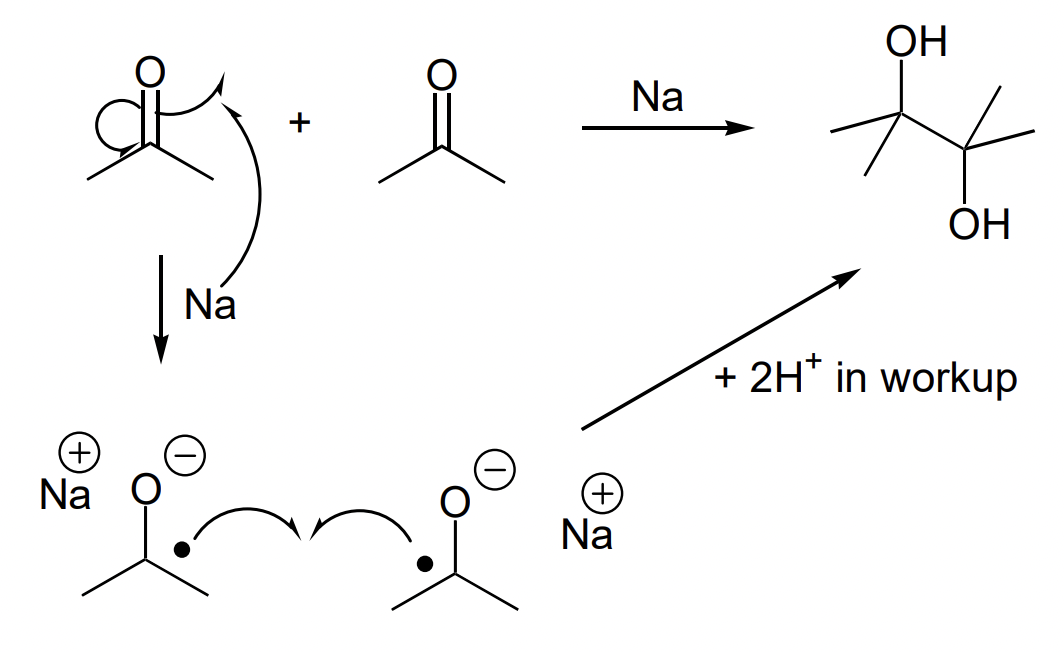
describe the pinacol coupling reaction
= synthesis of 1,2 diols
= Na OR Mg
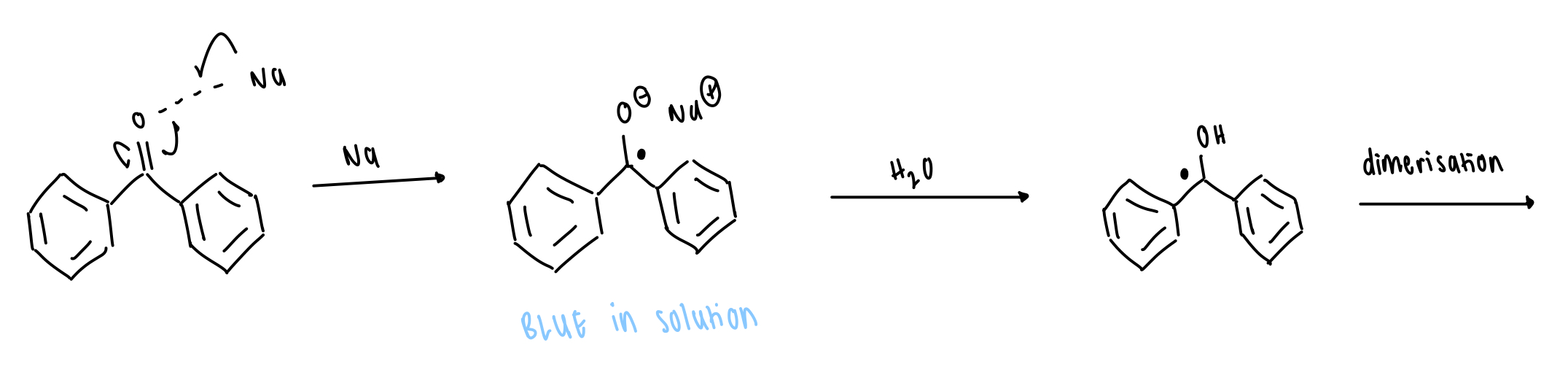
what is the application of benzophenone pinacol coupling
sodium radical salt = absence of water = blue
radical alcohol = presence of water = colourless
= indicator of water
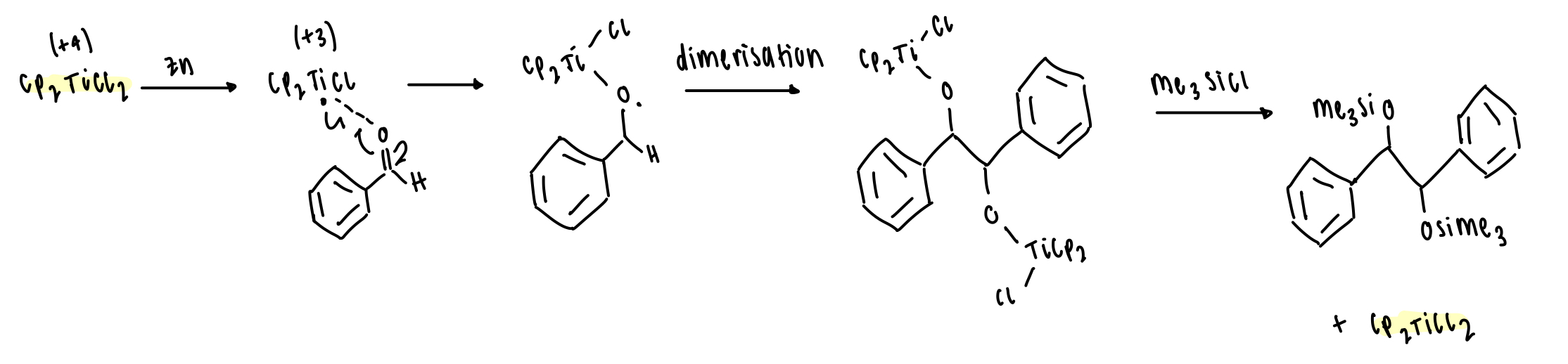
describe pinacol coupling with Ti and Zn
Ti = low VE = Lewis acid via single electron transfer with Zn
describe carbenes
neutral sp2 carbons with 2 non-bonding electrons
two types:
singlet = non-bonding electron pair in sp2 orbital
= empty orbital = Lewis acid
triplet = 1 electron in sp2 orbital, 1 electron in p orbital
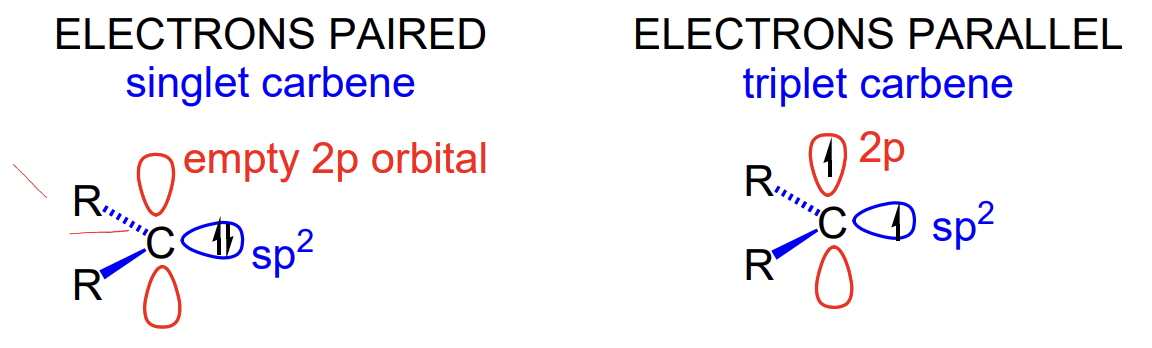
what controls carbenes tending to singlet/triplet states
steric effects/bond angles
small groups = small dihedral angle (between R) = singlet favoured
large groups = large dihedral = triplet favoured
solvent
polar solvent = stabilises singlet state by resonance
substituents
EDG/∏-donors (lone pairs N, O, P, S) = stabilise empty orbital by resonance = singlet favoured
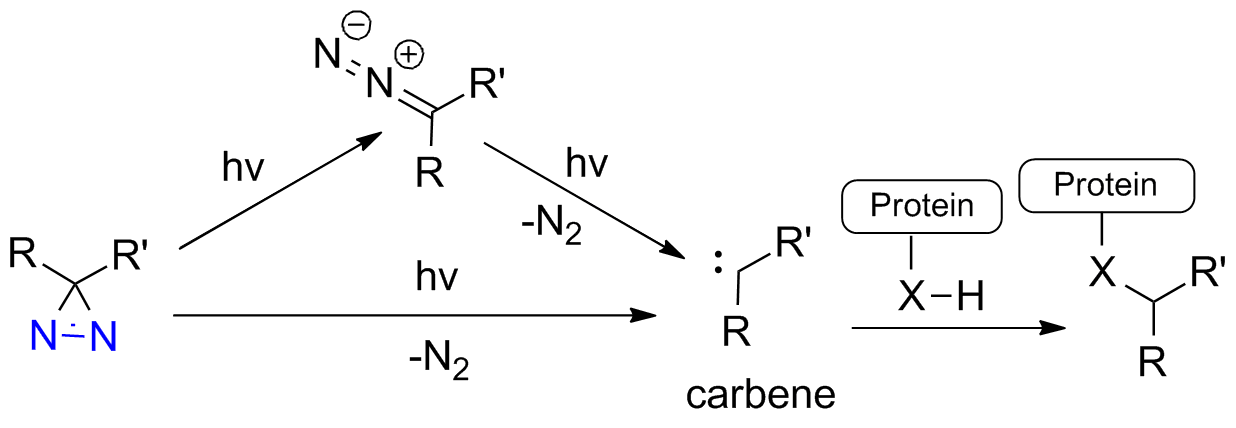
how can carbenes be generated?
generation from diazo compounds R2C=N=N
generation from tosylhydrazones = Bamford-Stevens reaction
a-eliminaation from 1,1-dihaloalkanes
describe carbene generation from diazo compounds
most common
thermally OR photolytically
driving force = loss of small, stable molecule
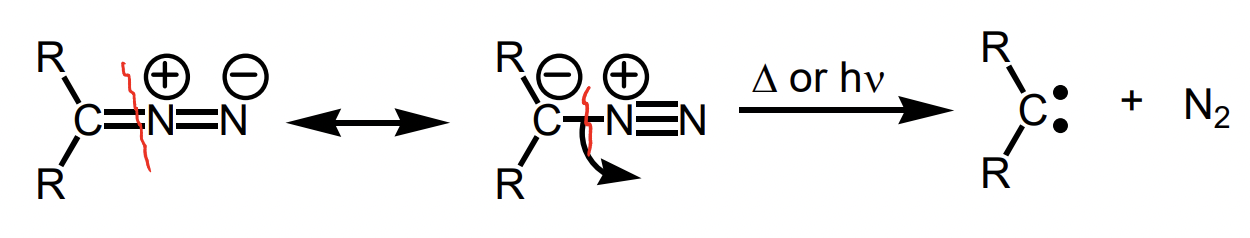
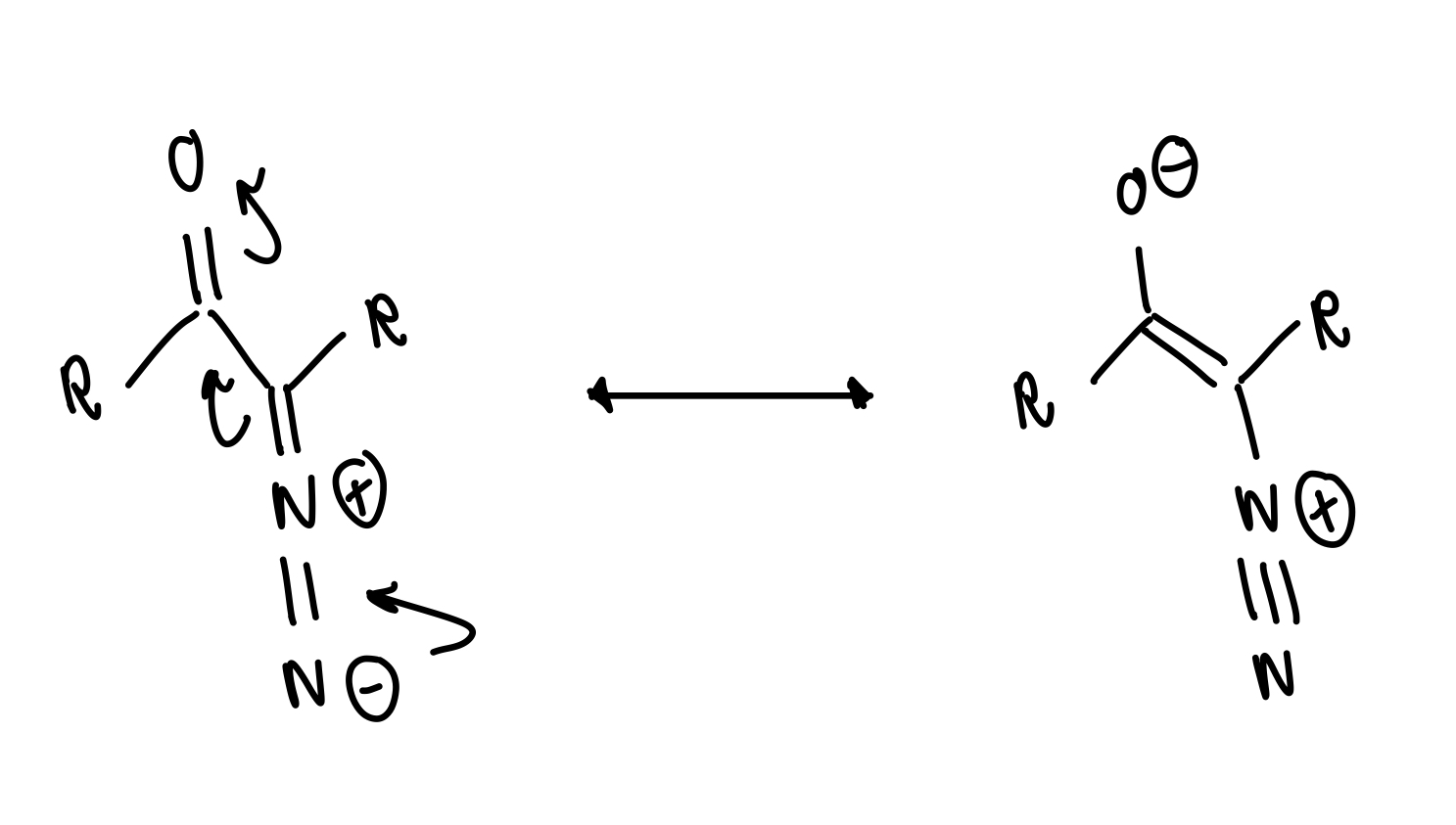
what is a stable form of diazo compounds
α-diazo-carbonyl
describe carbene generation from tosylhydrazones (Bamford-Stevens reaction)
ketone → tosylhydrazone → diazo → carbene
draw the entire Bamford-Stevens reaction (carbene generation)
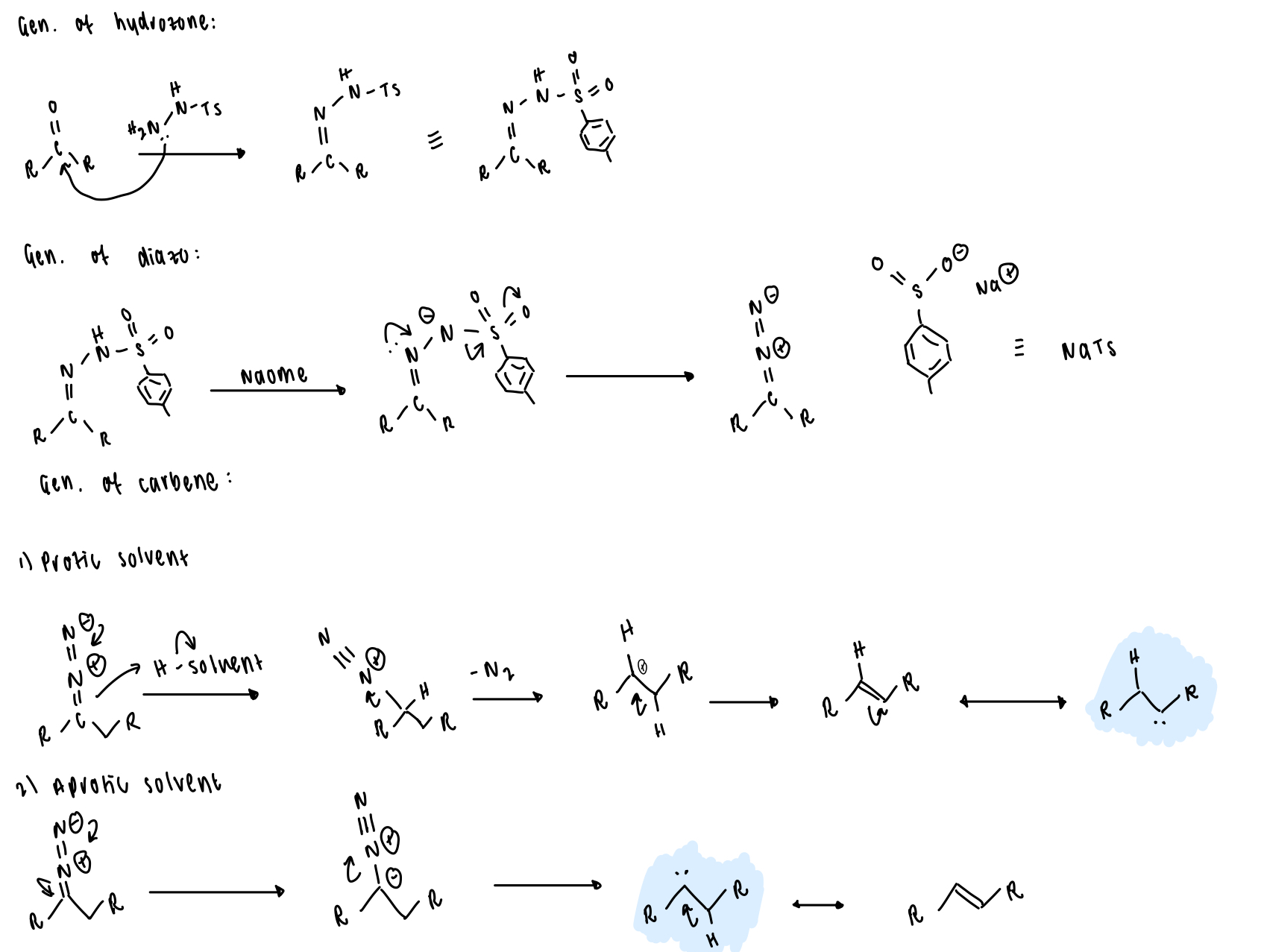
what is an alternative SM for Bamford-Stevens reactions?
diazirines
define a carbenoid
= metal complexed carbenes
= more stable
describe carbene generation by a-elimination from 1,1-dihaloalkanes
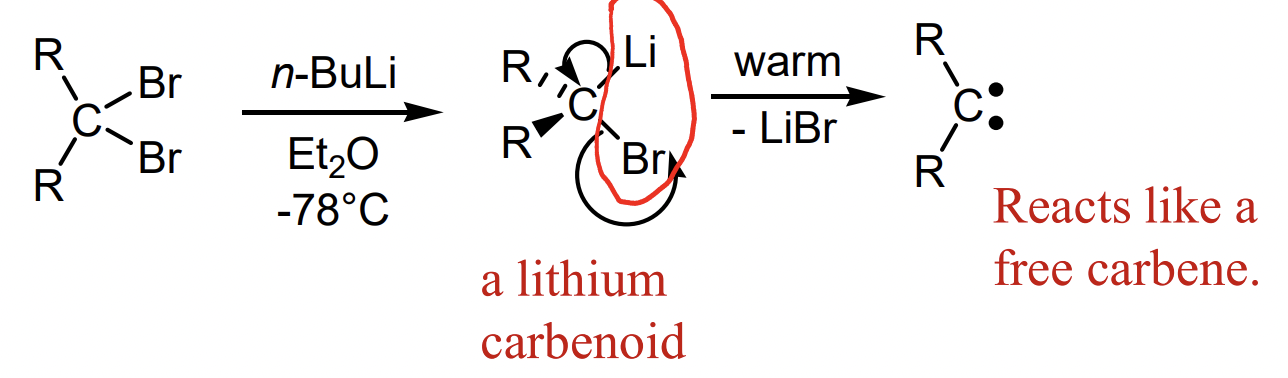
describe carbene generation from chloroform
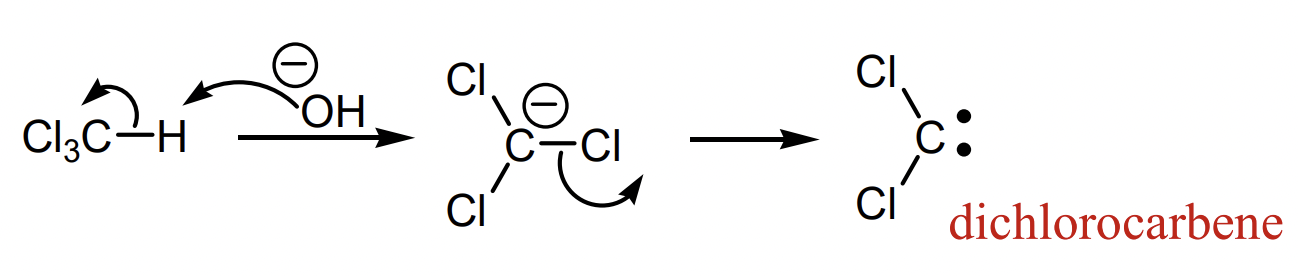
what are the different reactions of carbenes
cycloproponation
carbene insertion into single bonds
carbene rearrangement reactions
what is the most common carbene configuration
singlet
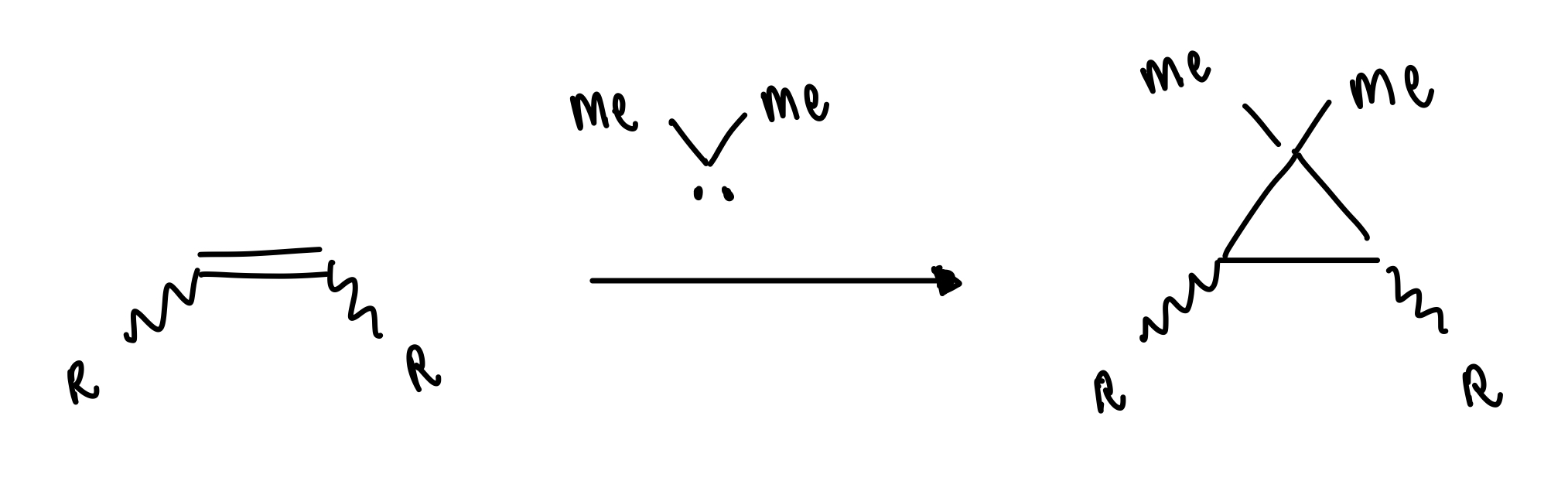
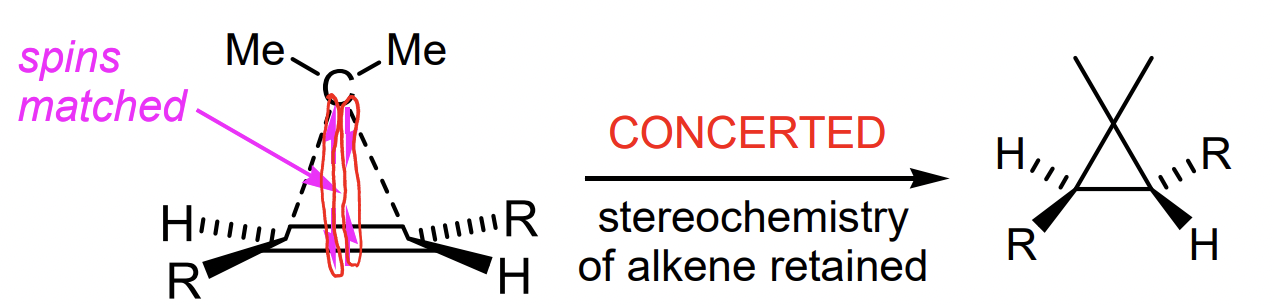
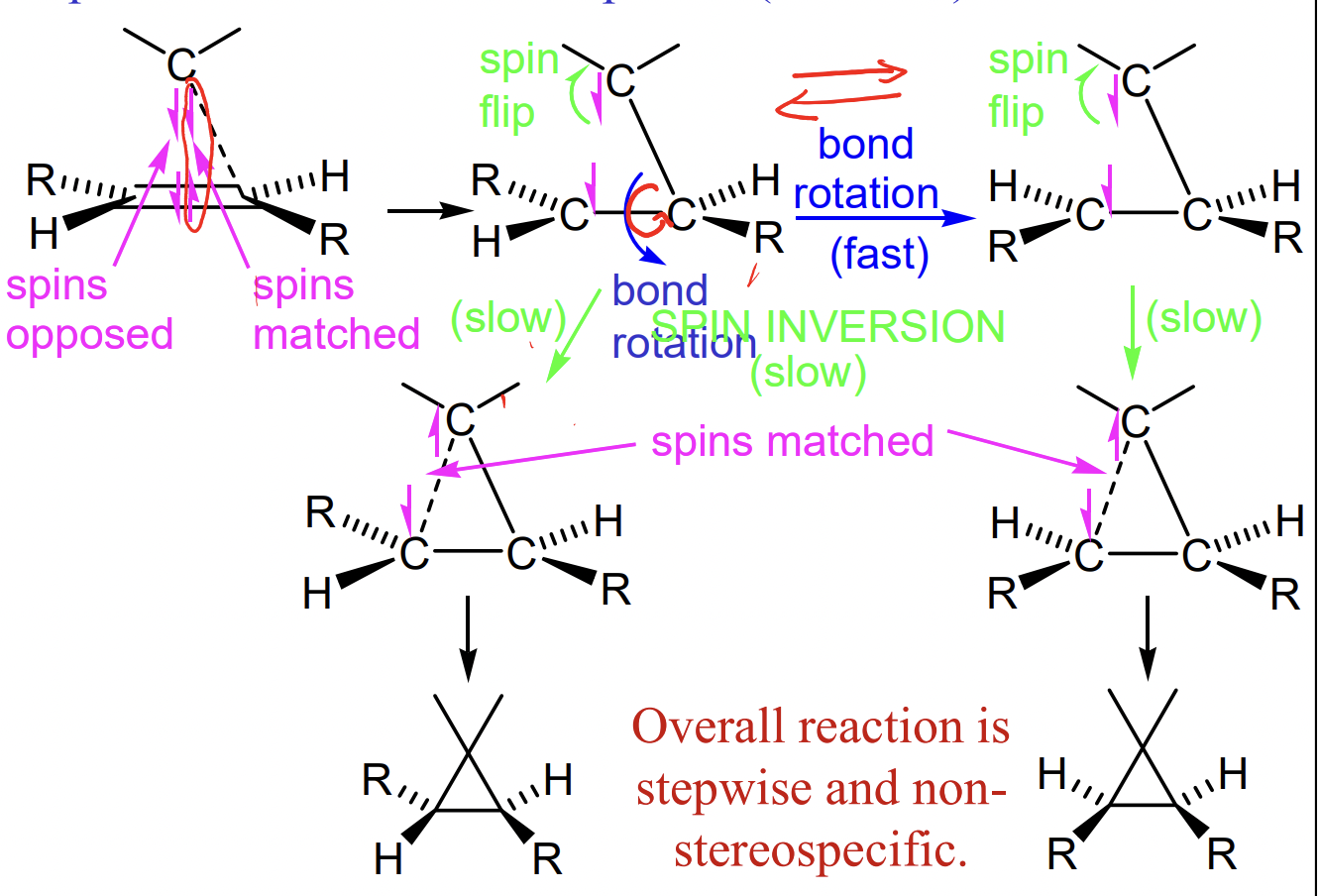
describe carbene cycloproponation
= insertion into an alkene
singlet carbene = stereospecific syn addition major
triplet carbene = non-stereospecific (diradical) addition
describe singlet carbene cycloproponation
spins matched = fast
describe triplet carbene cycloproponation
spin flip required = slow
bond rotation possible before spin flip
describe the Simmons-Smith reaction (carbenoid cycloproponation)
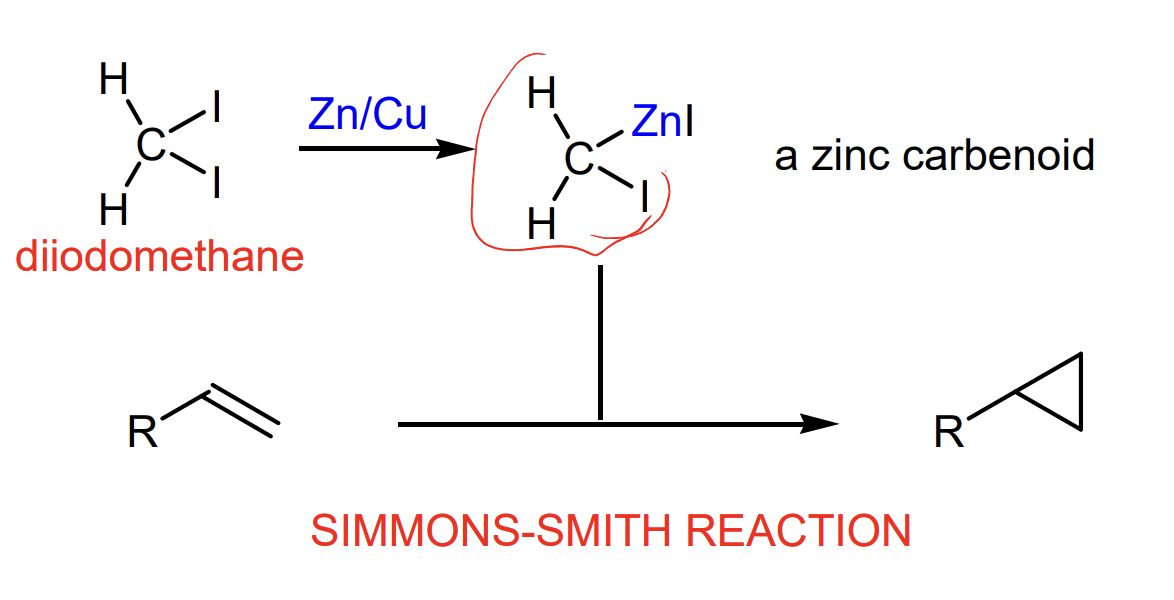
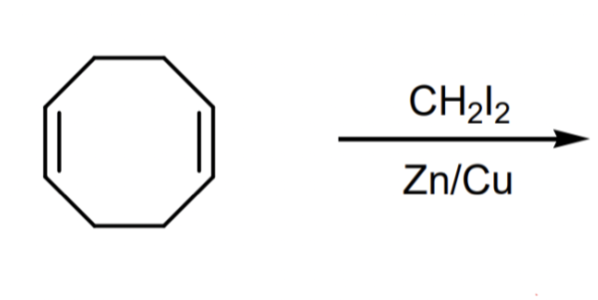
what is the product of this reaction
= formation of carbenoid
= carbenoid cycloproponation (Simmons-Smith)
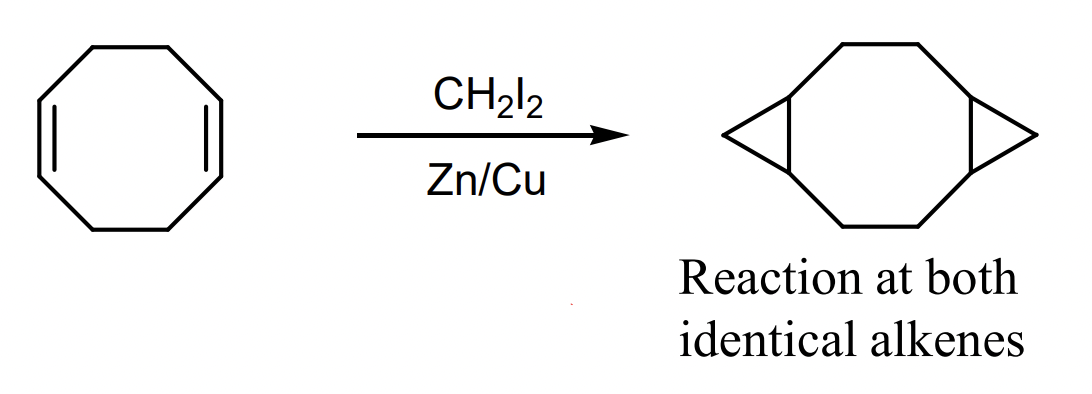
what kind of alkenes are favoured by Simmon-Smith reactions?
electron rich