Chemistry Unit 14: Thermochemistry
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Thermochemical equation
Chemical equation including energy change in reaction.
Enthalpy (H)
Heat content of a substance at constant pressure.
ΔH
Change in enthalpy during a reaction.
Exothermic Reactions
Reactions that release energy, ΔH is negative.
Endothermic Reactions
Reactions that absorb energy, ΔH is positive.
Chemical Potential Energy
Stored energy in chemical bonds of substances.
Law of Conservation of Energy
Energy cannot be created or destroyed, only transformed.
Calorimetry
Measurement of heat changes in chemical processes.
Specific heat (c)
Heat required to raise 1g of substance by 1°C.
Heating curve
Graph showing temperature changes during phase transitions.
Molar heat of fusion (ΔHfus)
Energy required to melt a substance from solid to liquid.
Molar heat of vaporization (ΔHvap)
Energy required to vaporize a substance from liquid to gas.
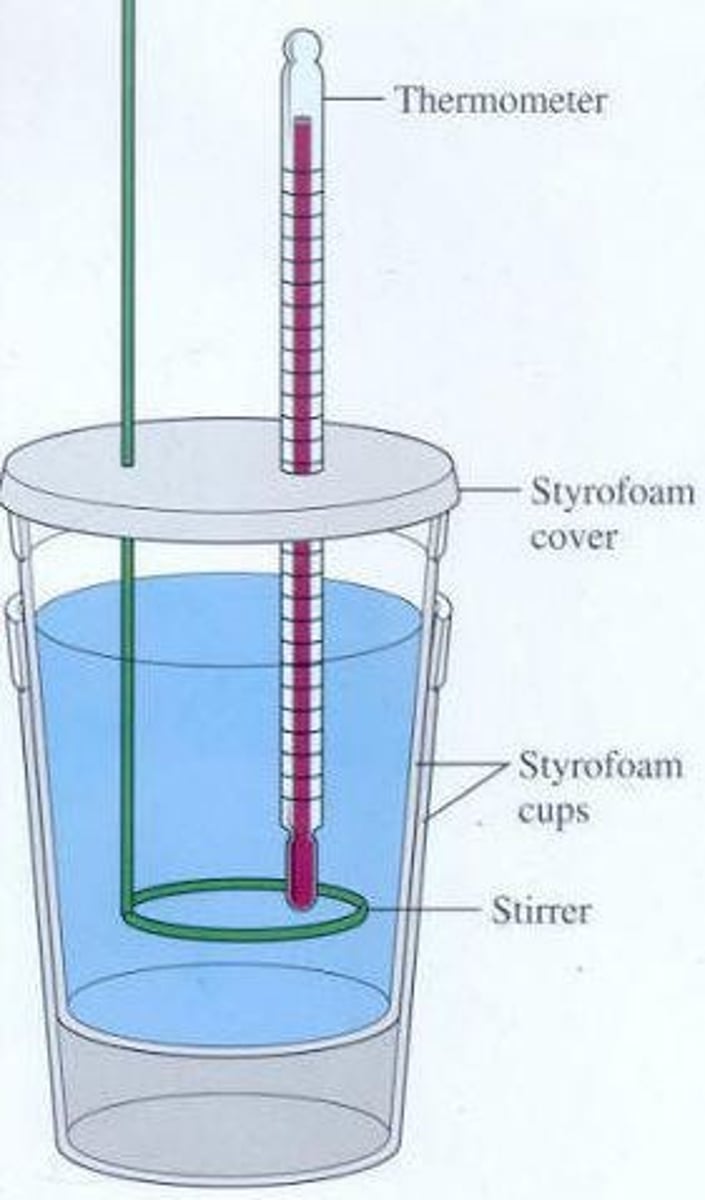
Coffee Cup Calorimeter
Constant-pressure calorimeter using water to measure heat.
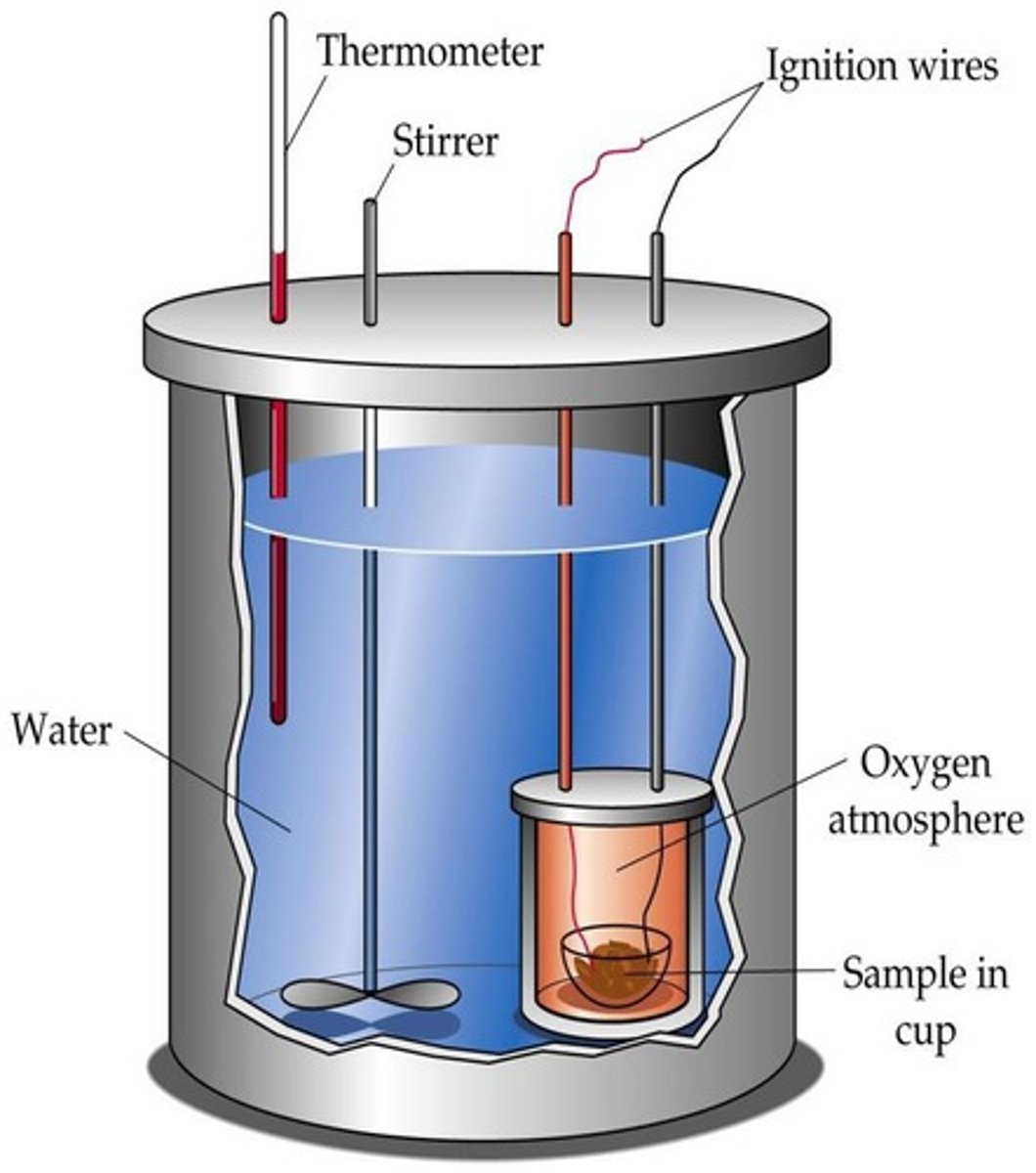
Bomb Calorimeter
Constant-volume calorimeter measuring heat from combustion.
Thermal equilibrium
State when two objects reach the same temperature.
Hess's Law
The overall enthalpy change in a reaction is equal to the sum of enthalpy changes for the individual steps in the process