Environmental Previous Exams
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
At what temp is water most dense
4 degrees celsius
individuals infected with which of the following pathogens can spread the disease via dormant cysts, but are only 5-10% likely to show symptoms
Giardia
Hydrobromic acid (HBr) is a strong acid. If sodium bromide (NaBr) is added to water at pH 7 and completely dissociates, what will happen to the pH
pH will not change
Which of the following is an example of an oppurtunistic pathogen
Legionella pneumophila
the Pka of 1-propanol (Ch3CH2CH2OH) is 16.9 at normal pH range ~(6-8) which can we assume is present at negligible concentration - protonated or deprotonated (CH3CH2CH2O-)
Deprotonated
What is the formula for the sulfate anion
SO4²-
which of the following pollutants is associated with agriculture
all (nitrogen, salts, phosphorous)
Which of the following parameters is used to quantify the amount of suspended solid particles in water
Turbidity
Chemical elements are defined by the atoms containing a certain number of what
Protons
Which of the following statements apply to endocrine disruptors
can have toxicological effects even at low concentrations, can interfere with hormone function
Which of the following is regulated by a secondary drinking water standard, as part of the Safe Drinking Water Act
taste and odor
What is the main goal of coagulation in drinking water treatment
To neutralize surface charge of colloidal particles
How do granular media filters remove colloidal particles from water
the colloids collide with media particles and stick to them
This molecule is an example of what
PFAS
Contamination from agricultural runoff or road salts are example of what
nonpoint source pollution
Which of the following waterborne illnesses is caused by a blood fluke
Schistosomiasis
Covalent bonding involves the sharing of electrons among atoms
True
Which of the contaminants is known to bioaccumulate
Mercury (Hg)
Toluene is an example of what type of molecule
Aromatic, Organic
Primary standards of the safe water drinking act come in which two forms
Maximum contaminant levels and treatment techniques
Adjusting the flow ratio between return (RAS) and waste (WAS) lines in the activated sludge process affects which of the following
All (Ratio of BOD conversions to CO2 v. Biomass, Food to microbe ratio (F/M), Cell retention time (thetac)
The conversion of N2 gas to nitrogen-containing nutrients such as nitrate and ammonia is known as
Nitrogen Fixation
Which is a characteristic of combined chlorine disinfection
All (Involves both addition of free chlorine and ammonia, leads to fewer byproducts than free chlorine, provides a relatively long-lasting residual in the distribution system)
Which of the following is an alternative process to activated sludge for BOD removal
Membrane Bioreactor
Which organism is most resistant to inactivation by chemical oxidants
Cryptosporidium parvum cysts
An activated sludge process with high BOD removal ratio, high aeration requirements, and poor settle ability of solids is characteristic of what type of cell (solids) retention time
High (>6d)
What role do anerobic digestors play in wastewater treatment
None (removal of recalcitrant dissolved organic compounds, disinfection of treated waste water, ammonia removal prior to activated sludge treatment)
Which of the following can be removed from wastewater by precipitating as a solid, followed by sedimentation
Phosphorous
What type of reactor can be used to determine the rate constant (k) of a chemical reaction
Batch reactor
If only organic compounds are present with no external electron acceptors such as O2 or SO4²-, which type of metabolism could microbes use to obtain energy
Fermentation
A water sample is collected from a polluted river and analyzed for BOD5 using the appropriate methods. Which of the following would affect the result
The biodegradability of the organic compounds in water
Why does organic nitrogen contribute minimally to BOD5
it takes longer than 5 days for bacteria to begin using it as an electron donor
Consider a point source discharging oxygen-demanding wastes into a river. Which of the following can affect the distance of the critical point (DO min) downstream from the discharge point
All (Stream Velocity, Altitude, Turbulent white water v. non-turbulent flow)
Which of the following is true of heterotrophs
All (They require preexisting organic compounds (reduced carbon), They obtain energy from thermodynamically favorable redox reactions, They contribute to oxygen depletion in waters with high BOD)
A conservative (unreactive) compound is briefly injected into the influent of an ideally-performing plug flow reactor which of the plots shows the concentration-time profile that will be observed in the reactor effluent
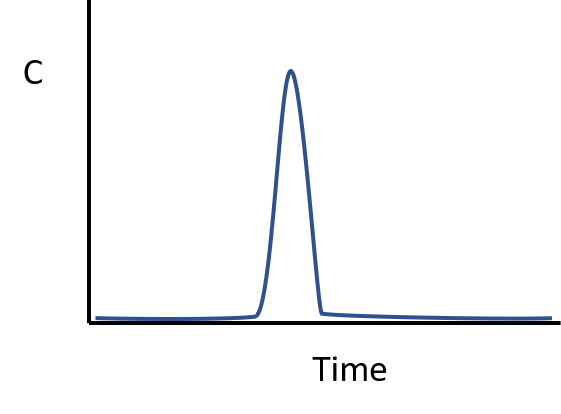
Which of the following is a first-order rate expression for a chemical degradation reaction
dC/dt = -kC
Consider a lake with high alkalinity that begins to be contaminated by acid rain which of the following will happen
The pH will decrease only slightly at first
Biological treatment of wastewater using aeration/aerobic conditions followed by anaerobic conditions is a common way to remove what
Nitrogen Compounds
What is the defining quality of an autotroph
They obtain carbon for synthesis from CO2
Which is a water disinfectant that inactivates microorganisms by directly causing DNA mutations
Ultraviolet radiation