Innate Immunity 1
1/106
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
107 Terms
Innate and adaptive immuniity
What happens if the body has no neutrophils (PMNs) or macrophages (MΦ)?
The body cannot mount an effective innate immune response and becomes highly susceptible to infections
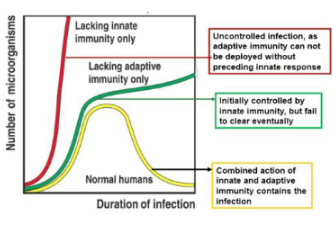
What happens if the body has neutrophils and macrophages but lacks T cells and B cells?
The innate immune system can still respond, but there will be no adaptive (specific) immune response, leading to limited long-term protection.
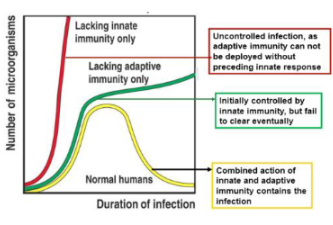
What is the importance of the innate immune system in relation to the adaptive immune system?
A strong innate immune response is essential because it activates and shapes the adaptive immune response
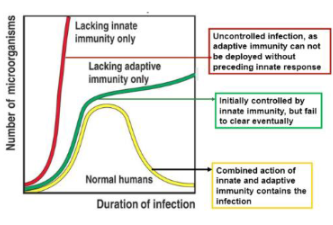
What occurs if the innate immune response is weak?
The adaptive immune response will also be weak or feeble, as it depends on signals from the innate system
How does innate immune recognition influence overall immunity?
Recognition of pathogens by innate immune cells sets the stage for an effective overall immune response.
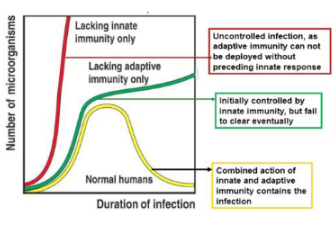
What are the three broad roles of the innate immune response to viruses?
To limit viral entry, translation, replication, and the release of new virions.
To identify and eliminate infected cells.
To accelerate the development of a targeted adaptive immune response.
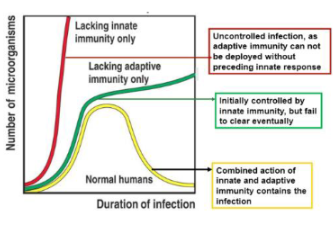
How does the innate immune system detect viral infections?
It uses pattern recognition receptors (PRRs) on the cell surface, in the cytosol, and in endosomes to recognise pathogen-associated molecular patterns (PAMPs).
What happens when PRRs detect viral PAMPs?
An inflammatory cascade is triggered, leading to controlled cell death in infected cells to limit viral spread.
What is the purpose of controlled cell death during a viral infection?
It helps to remove infected cells before they can produce and release new infectious virions
What can result from excessive activation of the innate immune system?
Severe systemic inflammation, which can contribute to conditions such as long COVID or even death.
How does the innate immune response support the adaptive immune system during viral infection?
By providing signals and inflammatory mediators that enhance the activation and targeting of T and B cells
Coronavirus: mucosal barrier
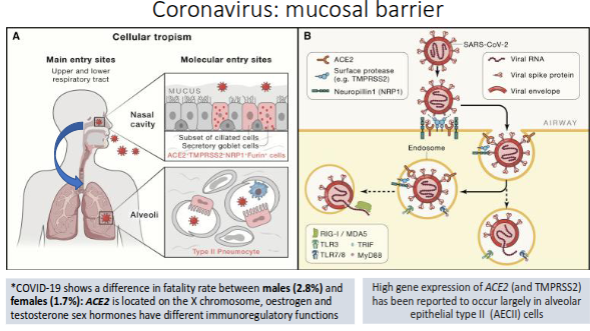
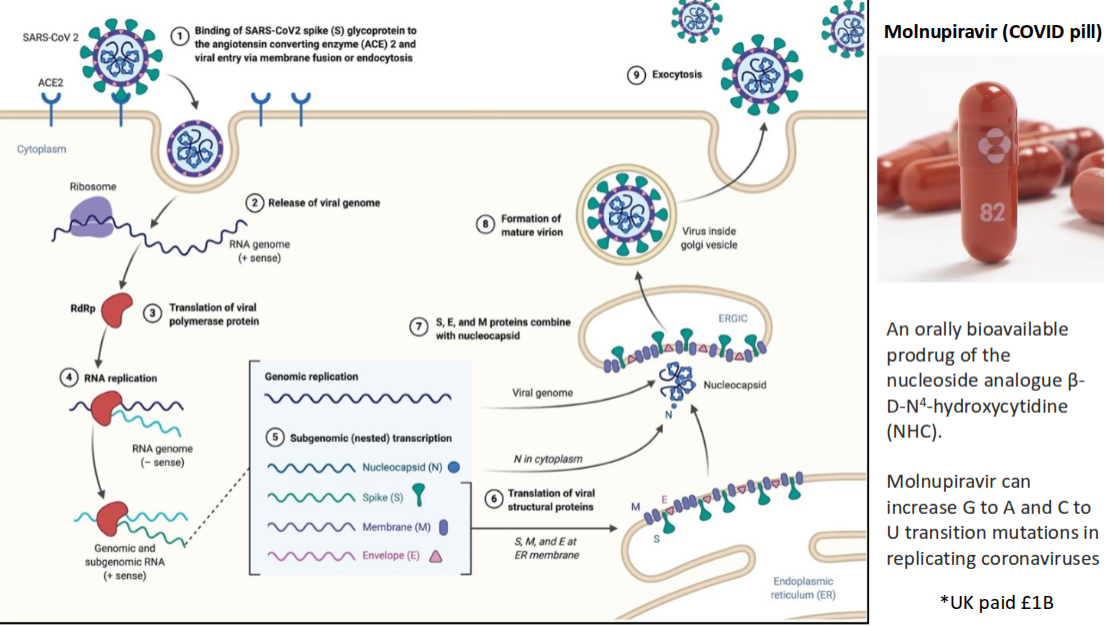
How does the Molnupiravir (COVID pill) work?
What is the role of the epithelial barrier in innate immunity?
It serves as the body’s first line of defence, preventing pathogen entry through physical, chemical, and immune mechanisms.
How do glycoproteins and glycolipids contribute to mucosal defence?
They prevent microorganisms from adhering to epithelial surfaces
What is the function of mucus flow and beating cilia in the lungs?
They help clear trapped microbes and debris from the respiratory tract
Which cells in the alveoli activate alveolar macrophages?
Alveolar epithelial type II cells (AECII).
What is the function of antimicrobial peptides (AMPs) in the lung mucosa?
They directly kill or inhibit the growth of microbes.
What is the role of tight and adherens junctions in the epithelial barrier?
They maintain the integrity of the epithelial layer, preventing pathogen penetration between cells.
tight junctions act as a barrier to prevent the free passage of ions, water, and other molecules through the spaces between cells (paracellular pathway)
adheren junctions mediate and stabilise cell-to-cell adhesion, providing mechanical strength and linking cells together.
What do pattern-recognition receptors (PRRs) on epithelial cells do?
They detect pathogen-associated molecular patterns (PAMPs) and trigger immune responses.
What is Immunoglobulin A (IgA), and how is it related to mucosal immunity?
IgA is an antibody important for mucosal defence; it is part of the adaptive immune response but can also be received passively from the mother.
Where are defensins particularly important?
In barrier organs such as the lungs, skin, and gut.
What are human defensins?
Small cationic peptides of 29–35 amino acids containing six invariant cysteine residues
they protect the host by killing microbes, modulating the immune system, and playing roles in other processes like fertility, wound healing, and development.
They have direct antimicrobial effects by disrupting microbial membranes and can also inhibit viral infection by targeting viral particles or interfering with cellular processes.
Which type of defensins are produced by lung epithelial cells?
β-defensins (beta-defensins).
What types of microorganisms can human defensins kill?
A wide range of bacteria and some enveloped viruses.
Name some bacteria targeted by human defensins.
E. coli, Streptococcus pneumoniae, Pseudomonas aeruginosa, and Haemophilus influenzae.
Which viruses are susceptible to human defensins?
Enveloped viruses such as herpesviruses and influenza virus.
How quickly do defensins act against pathogens?
They kill rapidly—often within minutes.
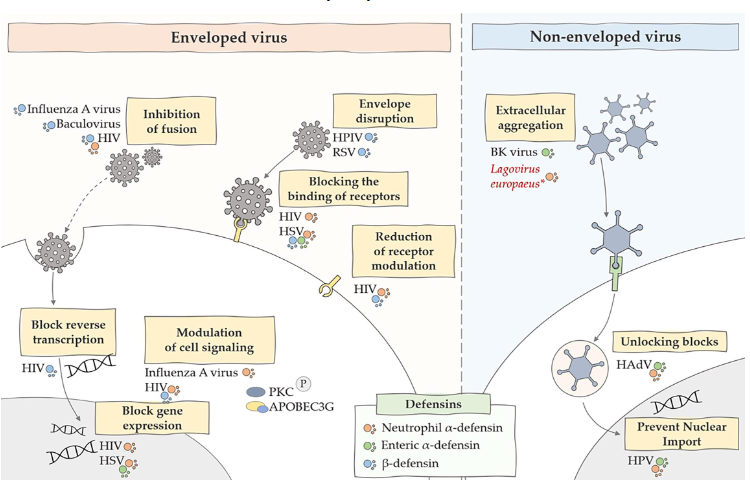
Antimicrobial peptides – Defensins and viruses
stimulated by pattern recognition receptors (PRRs) – in this case TLR3 detecting viral
pathogen- associated molecular pattern (PAMP) - double-stranded RNA
What are pathogen-associated molecular patterns (PAMPs)?
Conserved molecular structures found on microbes that are recognised by the innate immune system.
Why are PAMPs effective targets for immune recognition?
They are essential to microbial survival and therefore difficult for microbes to change or mutate.
What are pattern-recognition receptors (PRRs)?
Host receptors that detect PAMPs and trigger innate immune responses
How many Toll-like receptors (TLRs) and NOD-like receptor (NLR) genes are present in humans?
Humans have 10 TLRs and approximately 22 NLR genes
How significant is innate immunity across living organisms?
It is the only form of immunity in over 95% of living organisms.
In vertebrates, what proportion of protective efficacy is provided by innate immunity?
Around 80% of protective efficacy comes from innate immunity
Which animals have adaptive immunity?
Only jawed vertebrates possess adaptive immunity
What does the sea urchin genome reveal about innate immune diversity?
It encodes 222 Toll-like receptors (TLRs) and 203 NLRs, showing a highly expanded innate immune repertoire
Pattern Recognition Receptors
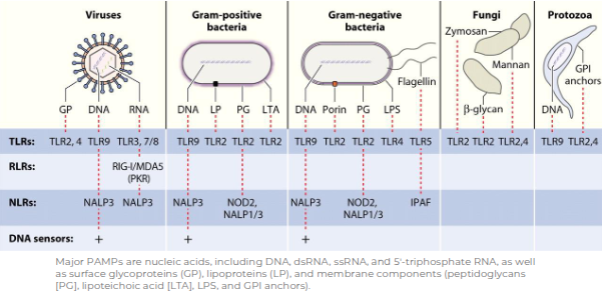
What does TLR stand for?
Toll-like receptor.
How many Toll-like receptors (TLRs) are found in humans?
10 TLRs
What is the main function of TLRs?
They act as recognition receptors for pathogen-associated molecular patterns (PAMPs).
What types of pathogens are detected by TLRs?
Bacteria, viruses, fungi, and parasites.
To which arm of the immune system do TLRs belong?
The innate immune system.
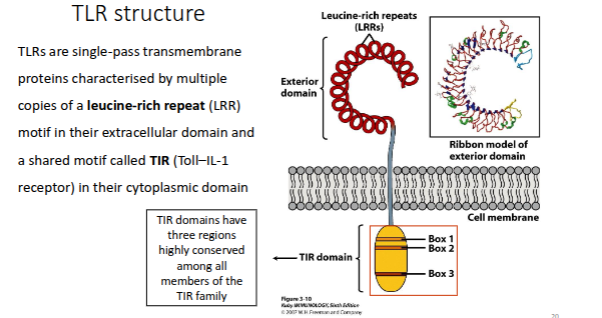
Describe the structure of TLRs
Wide-range of ligands
Domains all adopt horseshoe-
shaped structures built from LRR
motifOn ligand binding, two domains form an “M”-shaped dimer sandwiching the ligand molecule bringing the
transmembrane and cytoplasmic domains in close proximity and triggering a downstream signalling
cascade through TIR domain
How does Toll-like receptor (TLR) binding evoke an appropriate immune response?
By activating intracellular signalling pathways that lead to the production of antimicrobial molecules, chemokines, and cytokines.
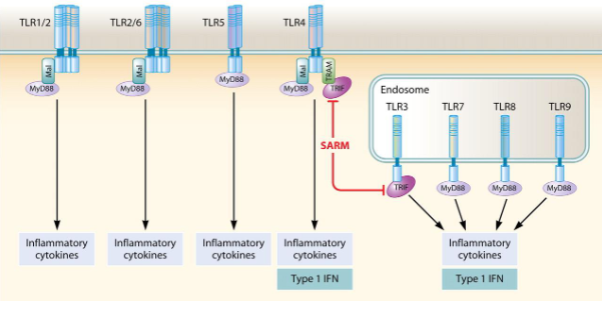
What are the two main signalling pathways triggered by TLR activation?
The MyD88-dependent pathway and the TRIF-dependent pathway.
Which adaptor protein is used by all TLRs except TLR3?
MyD88 (myeloid differentiation primary response 88).
What transcription factors are activated in the MyD88-dependent pathway?
NF-κB (p50/p65) and MAPK (mitogen-activated protein kinase).
What are the key outcomes of the MyD88-dependent pathway?
Production of defensins, iNOS, chemokines (e.g. CXCL8), and cytokines (e.g. TNF, IL-1, IL-6, IFNγ).
Which pathway primarily responds to viral infections via TLR3 and TLR4?
The TRIF-dependent pathway
What transcription factors are activated by the TRIF-dependent pathway?
Interferon regulatory factors (IRFs)
What is produced as a result of TRIF-dependent signalling?
Type I interferons, such as IFN-α and IFN-β
How do different Toll-like receptors (TLRs) activate immune responses through MyD88 and TRIF pathways?
Cell surface TLRs (TLR1/2, TLR2/6, TLR5, TLR4) signal mainly via MyD88, leading to production of inflammatory cytokines.
TLR4 can also signal through TRIF, inducing both inflammatory cytokines and type I interferons (IFNs).
Endosomal TLRs (TLR3, TLR7, TLR8, TLR9) detect viral nucleic acids:
TLR3 signals via TRIF → type I IFN.
TLR7, TLR8, and TLR9 signal via MyD88 → inflammatory cytokines and type I IFN.
SARM negatively regulates TRIF-dependent signalling.
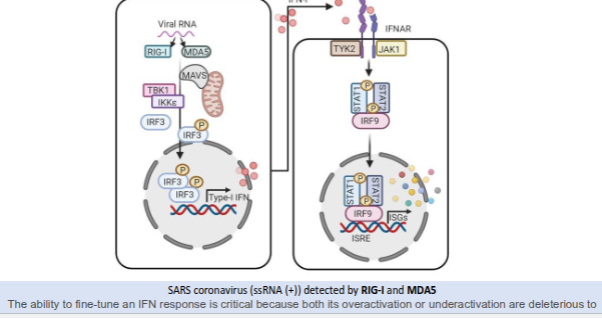
How does the innate immune system detect SARS coronavirus and trigger an interferon (IFN) response?
RIG-I and MDA5 detect viral positive-sense single-stranded RNA (ssRNA⁺).
Detection activates MAVS, which recruits TBK1 and IKKε, leading to phosphorylation of IRF3.
Activated IRF3 enters the nucleus and induces type I interferon (IFN-α/β) production.
Type I IFNs bind to the IFNAR receptor, activating JAK1 and TYK2.
This triggers phosphorylation of STAT1 and STAT2, which form a complex with IRF9.
The STAT1–STAT2–IRF9 complex activates interferon-stimulated genes (ISGs), establishing an antiviral state.
Balanced IFN activation is crucial—overactivation or underactivation can both cause harm.
What are Type I interferons released in response to?
They are released in response to viruses, bacteria, parasites, and tumour cells.
How do Type I interferons influence the immune system?
They shape both the innate and adaptive immune responses
by inducing an antiviral state in cells, stimulating both innate and adaptive immune responses, and modulating their outcomes based on the context of the infection. They activate innate immune cells like natural killer (NK) cells and dendritic cells (DCs), enhance antigen presentation, and boost adaptive immunity by promoting T and B cell activity, antibody production, and T cell expansion. However, excessive IFN-I can cause immunopathology or immunosuppression in certain chronic or bacterial infections.
How do Type I interferons signal?
They act in an autocrine and paracrine manner by binding to the Type I interferon receptor (IFNAR).
Give examples of Type I interferons.
Interferon-α (alpha) and interferon-β (beta).
What cells are stimulated by Type I interferons to produce an antiviral response?
Macrophages and natural killer (NK) cells.
Which antiviral proteins are induced by Type I interferons?
Protein kinase R, 2’,5’-oligoadenylate synthetase, and Mx proteins
What is the main effect of proteins induced by Type I interferons?
They inhibit viral replication.
What are cytokines?
Small signalling proteins that mediate communication between immune cells and regulate inflammation, fever, and tissue responses.
Name three key pro-inflammatory cytokines and their main effects.
IL-1: Causes fever and tissue damage.
IL-6: Causes fever.
TNF: Induces apoptosis (cell death).
Which immune cells are activated by cytokines such as IL-1, IL-6, and TNF?
Tissue-resident macrophages
What is the function of granulocyte macrophage colony-stimulating factor (GM-CSF)?
It stimulates the production and activation of granulocytes and macrophages
What are chemokines?
A subset of cytokines that direct the migration (chemotaxis) of immune cells to sites of infection or inflammation
Which chemokine attracts neutrophils (PMNs)?
Interleukin-8 (IL-8).
Which chemokine attracts monocytes from the blood?
Monocyte chemoattractant protein-1 (MCP-1).
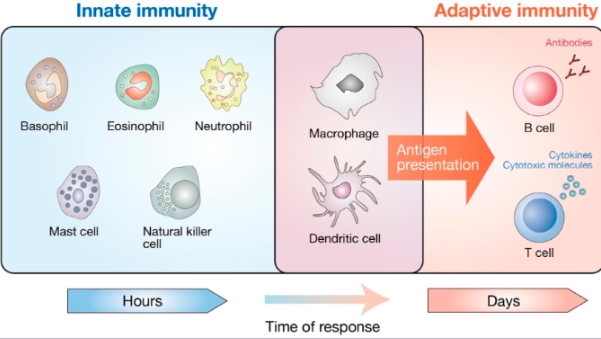
What are the main cell types of the innate immune system?
Neutrophils, monocytes/macrophages, dendritic cells, natural killer (NK) cells, mast cells, and eosinophils.
What proportion of leukocytes are neutrophils, and what is their main function?
50–70% of leukocytes; they perform phagocytosis and drive inflammation.
What proportion of leukocytes are monocytes/macrophages, and what are their key roles?
5–7% of leukocytes; they carry out phagocytosis, promote inflammation, activate T cells, and aid in tissue repair
What is the primary function of dendritic cells?
Phagocytosis, antigen presentation, and activation of naïve T cells, linking innate and adaptive immunity.
What percentage of leukocytes are mast cells, and what do they do?
About 0–1%; they release inflammatory mediators and contribute to allergic responses and inflammation.
What proportion of leukocytes are eosinophils, and what are their main functions?
2–4% of leukocytes; they are involved in inflammation and defence against parasites.
What is the principal role of natural killer (NK) cells?
They kill virally infected and tumour cells, contributing to both innate and adaptive immunity
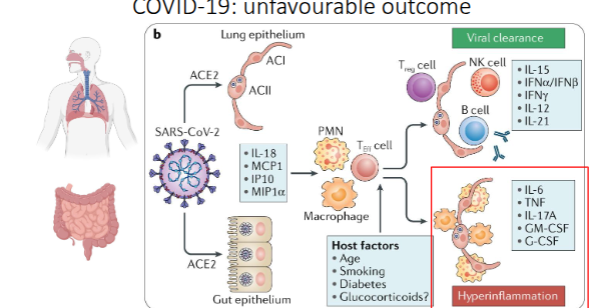
Coronavirus - lung innate immune cells
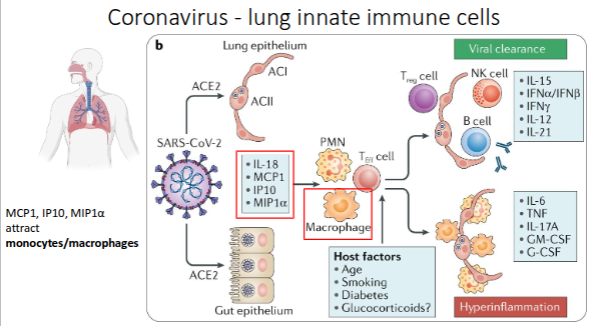
What are tissue-resident macrophages?
Long-lived macrophages that permanently reside in specific tissues, providing local immune defence and homeostasis
What are microglia, and where are they found?
Microglia are the brain’s resident macrophages, making up 10–15% of all brain cells and acting as the main immune defence in the central nervous system (CNS).
What are Kupffer cells, and what is their function?
Kupffer cells are liver-resident macrophages that line the sinusoids and break down red blood cells (RBCs)
Where are alveolar macrophages located, and why are they highly active?
They are found in the lungs and are highly active due to constant exposure to airborne pathogens and particles
What are Langerhans cells, and where are they found?
Langerhans cells are dendritic cells found in the skin and mucosa that capture antigens and initiate immune responses.
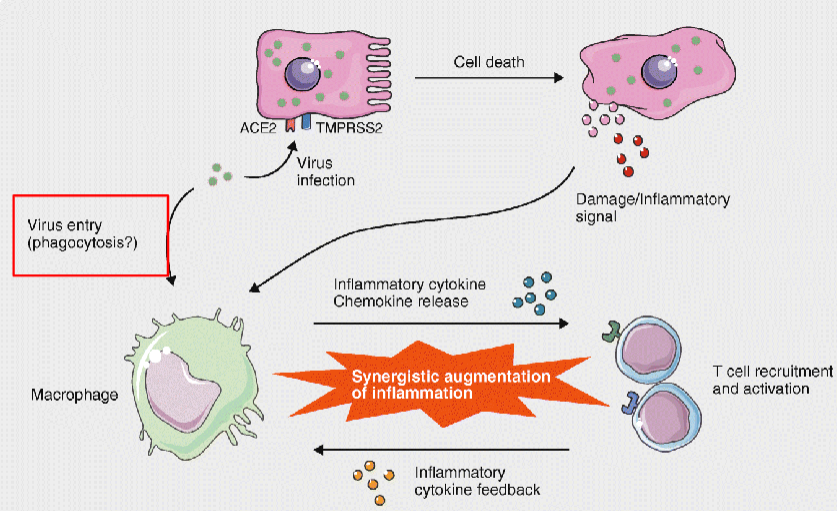
Although macrophages do not express ACE2, alveolar macrophages may uptake viral RNA
through phagocytosis and the degradation of virus-infected cells
What are the two main mechanisms of phagocytosis?
Complement-mediated phagocytosis – pathogens are coated with complement proteins, promoting uptake by phagocytes.
Antibody (Fc receptor)-mediated phagocytosis – antibodies (e.g. to SARS-CoV-2 spike protein) opsonise virus particles, enabling macrophages to engulf them via Fc receptor-mediated endocytosis once adaptive immunity is activated.
Natural killer (NK) cell activation
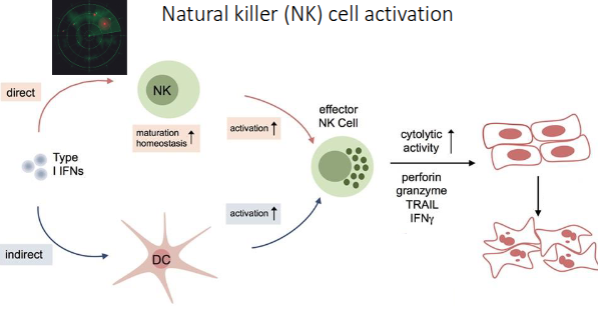
What is the primary role of natural killer (NK) cells?
NK cells provide rapid immune responses to destroy virus-infected cells and detect tumour formation.
How do NK cells recognise their targets?
They detect stressed or abnormal cells without the need for antibodies or MHC, allowing a much faster immune reaction
What cytokines do NK cells secrete, and what are their functions?
IFNγ (interferon gamma): Activates macrophages.
TNFα (tumour necrosis factor alpha): Stimulates dendritic cell maturation and inflammation.
What types of receptors regulate NK cell activity?
Inhibitory receptors (prevent killing of healthy cells) and activating receptors (trigger killing of infected or stressed cells)
What has research shown about NK cell levels in severe COVID-19 cases?
Severe COVID-19 patients have reduced peripheral NK cell counts compared with mild cases and healthy controls.
Why might children have milder COVID-19 symptoms?
Children tend to have higher NK cell counts, which contribute to more effective early viral control
What triggers dendritic cell (DC) activation and maturation?
Binding of Toll-like receptors (TLRs) to pathogen-associated molecular patterns (PAMPs).
What changes occur when DCs mature?
They increase surface expression of MHC Class II and co-stimulatory molecules needed for T-cell activation.
Where do activated dendritic cells migrate after activation?
To lymphoid tissue, where they present antigens to helper (CD4⁺) and cytotoxic (CD8⁺) T cells.
What happens when naïve T cells interact with activated dendritic cells?
Naïve CD4⁺ and CD8⁺ T cells differentiate into subsets such as Th1, Th2, Th17, T follicular helper (TFH), or regulatory T cells, initiating adaptive immunity.
How do T follicular helper (TFH) cells contribute to immune defence?
They help B cells differentiate into antibody-secreting plasma cells.
What has been observed about dendritic cells in COVID-19 patients?
There is an increased abundance of activated DCs in the lungs compared with healthy controls.
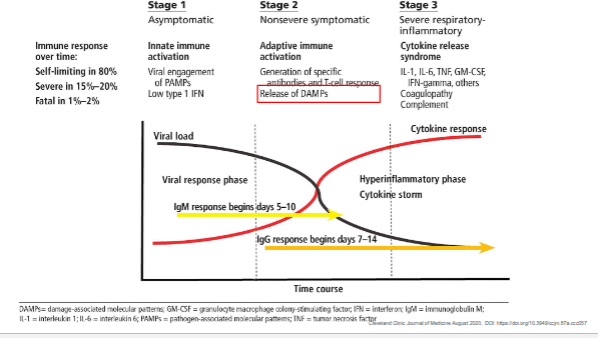
What are the three stages of immune response progression in viral infection, and what occurs in each stage?
Stage 1 – Asymptomatic (Innate immune activation):
Viral engagement of PAMPs triggers a mild type I interferon (IFN) response.
Infection is self-limiting in ~80% of cases.
Stage 2 – Non-severe symptomatic (Adaptive immune activation):
B and T cells generate specific antibodies and cytotoxic responses.
IgM appears around days 5–10, IgG around days 7–14.
DAMPs (damage-associated molecular patterns) are released, amplifying inflammation.
Stage 3 – Severe respiratory-inflammatory (Cytokine release syndrome):
Excessive production of cytokines (IL-1, IL-6, TNF, GM-CSF, IFNγ) causes a cytokine storm, coagulopathy, and potential respiratory failure.
This hyperinflammatory phase is responsible for severe or fatal disease in 15–20% of cases.
Key takeaway:
Early innate and adaptive balance is protective, but excessive cytokine activation leads to severe inflammation and tissue damage.
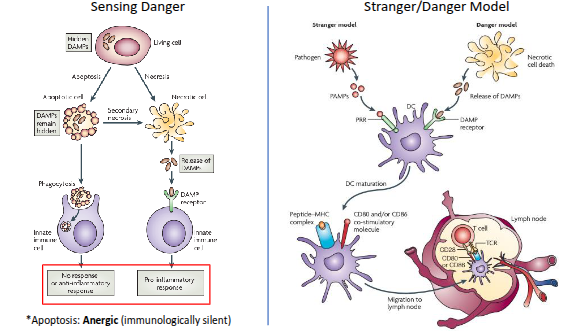
What is the difference between the stranger model and the danger model of immune activation?
Stranger model:
Proposed by Charles Janeway.
The immune system responds to non-self (“stranger”) molecules, recognised through pathogen-associated molecular patterns (PAMPs) via pattern-recognition receptors (PRRs).
Focuses on detecting microbial invaders.
Danger model:
Proposed by Polly Matzinger.
The immune system responds to signals of damage or stress—called damage-associated molecular patterns (DAMPs)—rather than to non-self alone.
Emphasises recognition of cell injury or tissue danger, even in the absence of infection.
Summary:
Stranger model → Detects foreign pathogens.
Danger model → Detects tissue damage or stress.
COVID-19: unfavourable outcome